Question
Question: An organic compound A having molecular formula \[{C_6}{H_6}O\] gives a violet colour with neutral \[...
An organic compound A having molecular formula C6H6O gives a violet colour with neutral FeCl3 solution. A on treatment with CO2 and NaOH at 400K under pressure gives B on acidification gives a compound C. The compound C reacts with acetyl chloride to give D which is a popular pain killer. Deduce structure of A, B, C and D.
Solution
Phenol react with CO2 and NaOH at 400K under pressure gives Salicylic acid (i.e. Kolbe–Schmitt reaction)
Complete step by step answer:
Given question can be represented as:
A+FeCl3→ Violet colour
ACO2,NaOH400KBAcidificationCAcetyl ChlorideD
Here the formation of a given violet compound on reaction withFeCl3 i.e. first step confirms that the given compound has a phenolic group. Second reaction of A to form B, C and then to D is the Kolbe-Schmitt reaction.
Step-1
In this step phenol reacts with FeCl3 and forms a violet colour compound. This reaction is an identification reaction for phenolic groups.
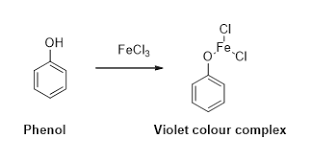
So the compound A is Phenol.
Step-2
This is a typical Kolbe Schmitt reaction in which an addition reaction occurs. This is a carboxylation reaction in which the sodium phenoxide forms first then it will react with carbon dioxide and form sodium salicylate. Sodium salicylate will form salicylic acid on acidification by sulphuric acid. The reaction occurs as the image showing below:
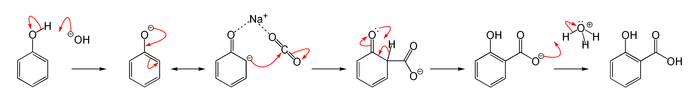
So the compound B is sodium salicylate and C is salicylic acid.
Step-3
On acetylation salicylic acid Acetyl salicylic acid formation takes place which is commonly known as aspirin i.e. a pain killer.
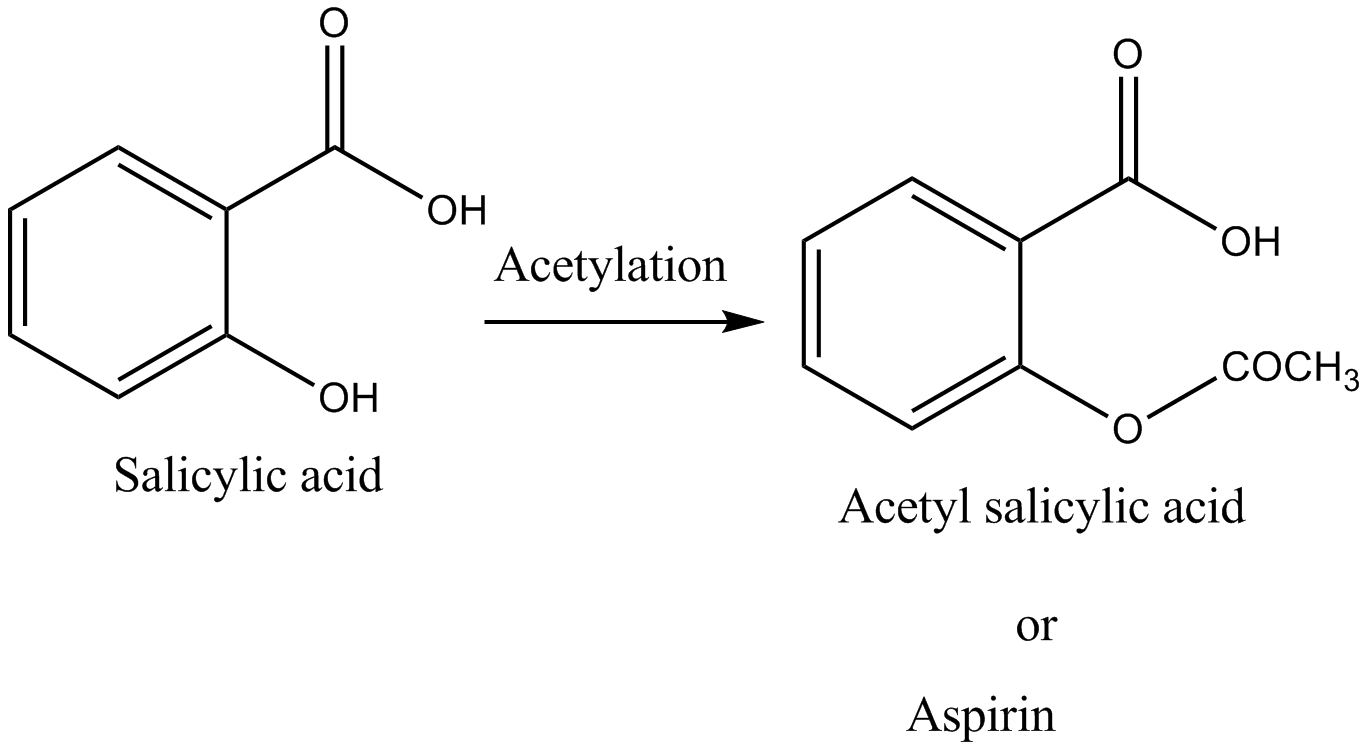
So, the correct answer is “Option D”.
Note: Just identify the starting product and the end product very carefully. Starting with phenol given the type of reaction it is and identification of the painkiller aspirin will help in finding the final product.
