Question
Question: An organic compound A having molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\) on...
An organic compound A having molecular formula C3H6 on treatment with aq. H2SO4 gives compound B which on treatment with HCl/ZnCl2 gives the compound C on treatment with ethanoic KOH gives back the compound A. Identify the compound A,B,C.
Solution
In the above question, we have to find the organic compound A, B and C. Since the molecular formula C3H6 which is a representation of alkene is given at the beginning, we can start solving the reaction occurred from the beginning. The first reaction is an additional reaction, followed by substitution of chloride ion against OH.
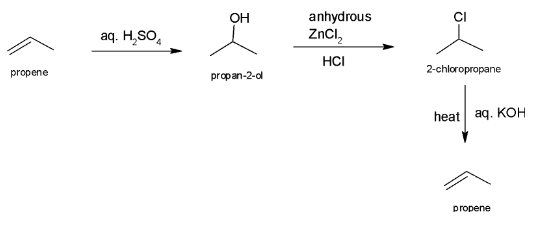
Complete step-by-step answer: In the above question, the compound A has molecular formula C3H6 which is of the form CnH2n which indicates that it is a propene.
So, A is propene.
When an alkene reacts with aq. H2SO4, H2O is added to the alkene. In this process, OH group gets attached to the carbon atom containing less number of H atom and H atom from H2O gets attached to carbon atom having more H atom.
So, propene reacts with aq. H2SO4 to form propan-2-ol. So, compound B is propan-2-ol.
The mixture of HCl and ZnCl2 form Lucas reagent which replace OH atom with Cl. Hence, compound C is 2-chloropropane.
When ethanoic KOH is added to 2-chloropropane it leads to elimination reaction and hence, propene is formed.
The whole reaction can be illustrated as:
Note: In these types of questions, we have to look where the compound molecular formula is given. If it is given in the beginning, we can start solving it as usual. If the molecular formula or molecule is given at the end, we must start solving the equation backward.
