Question
Question: An organic compound A, \[C_5H_8O\]; reacts with \[{{H}_{2}}O\], \[N{{H}_{3}}\] and \[C{{H}_{3}}COOH\...
An organic compound A, C5H8O; reacts with H2O, NH3 and CH3COOH as described above. A is:
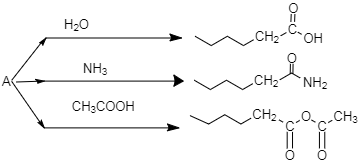
A.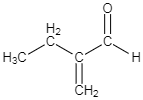
B.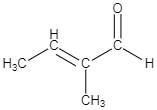
C.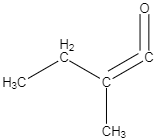
D.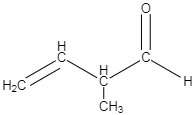
Solution
In case of water, ammonia and acetic acid, we can convert them into nucleophiles, just by removing one hydrogen atom, as it gains a negative charge, or in other words, it becomes electron rich in nature, that is , a nucleophile which attacks the required compound.
Nucleophiles are those substances which have high abundance of electrons and are looking for a compound where they could donate it.
Complete answer:
-In case of water, we know that water is a hydride of oxygen, so the proton donor would be the OH− in case of water. In case of NH3 the proton donor would be the NH2− group, and in case of acetic acid, the donor group would be acetate ion.
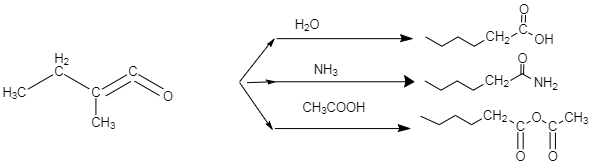
-In the above diagram we could see that the donor group attacks the double bond present between the two carbons. In case of water molecule, the OH− donates it two electrons to the double bond, as the oxygen attached to the terminal carbon, is making that double bond weak, or in other words, because of it high electronegativity, the oxygen pulls the shared pair of electron towards itself which in turn results in decrease in double bond character in the carbon-carbon bond, and the bond becomes more polar. This is when the nucleophile attacks the double bond, and it breaks, resulting in formation of a single OH− bond with the terminal carbon.
-Similarly, in case of ammonia, the NH2− group acts as a nucleophile, which attacks the double bond present between the two carbons. As a result, the NH2− group gets attached to that terminal carbon.
-In case of acetic acid, the acetate group acts as nucleophile, which attacks the double bond present between the two carbons. As a result, the acetate group gets attached to that terminal carbon. This mechanism can be expressed through the diagram, given below. This diagram only shows the case when water attacks the double bond. We can imagine the other mechanisms on the basis of the concept explained above.
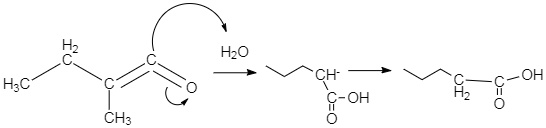
-As per the mechanism explained above, option C is the only option which could give the products which are mentioned in the question.
So the correct answer is option C.
Note:
-Oxygen is more electronegative than carbon, this is the reason why it gets to pull the shared pair of electrons towards itself.
-While writing the mechanism in organic reaction, the arrows are alway supposed to point from electron rich species to the electron deficient site, meaning, the arrows represent the movement of electrons and not the positive charge.
