Question
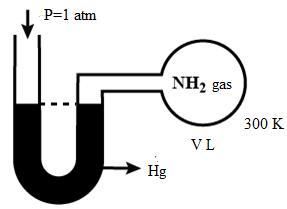
Question: An open ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in...
An open ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Initially the manometer shows no difference in mercury level in both columns as shown in the diagram.
After sparkling NH3 dissociated according to following given reaction
NH3(g)→21N2(g)+23H2(g)If pressure of NH3 decreases to 0.9 atm. Then the difference in mercury level is (assume temperature is constant during the entire process).
A. 228mmHg
B. 38mmHg
C. 76mmHg
D. 152mmHg
Solution
We can calculate the difference in the level of mercury using the pressures of ammonia, hydrogen, and nitrogen. We have to first calculate the pressures of hydrogen, nitrogen, and ammonia at equilibrium. From the pressures of each gas, we have to calculate the total pressure at equilibrium. We have to convert the value of total pressure at equilibrium into mmHg using the conversion factor.
Complete step by step answer:
According to the system, the atmospheric pressure would be given as 1atm which is nothing but the pressure of ammonia gas. Initially, there is no pressure exerted by the gas ammonia trapped in the container and mercury level is the same in two columns. Pressure of ammonia is equal to the atmospheric pressure and pressure of nitrogen and hydrogen is zero.
But when it is sparkling, the pressure of ammonia reduces to 0.9 atm. Thus, the variation in pressure of ammonia would be written as,
Initial pressure−Final pressure
1atm−0.9atm
0.1atm
Let us now consider the decrease in the pressure as X.
The given equation is,
NH3(g)→21N2(g)+23H2(g)
The pressure of ammonia is reduced to 1−X=0.9atm
The pressure of nitrogen gas is 0.5X=0.5×0.1=0.05atm
The pressure of hydrogen gas is 1.5X=1.5×0.1=0.15atm
By summing the pressure of ammonia, pressure of nitrogen, and pressure of hydrogen, we can now calculate the total pressure exerted within the system as,
Total pressure=Pressure of ammonia+Pressure of nitrogen+Pressure of hydrogen
Total pressure=PNH3+PN2+PH2
Let us now substitute the values in the expression to get the total pressure.
Total pressure =0.9atm+0.05atm+0.15atm
Total pressure =1.1atm
So, the total pressure exerted within the system is 1.1atm.
Thus, the increase in pressure of the system is because of the ammonia going through sparking which causes the increase in the levels of mercury.
So, the total pressure increased is calculated as,
ΔP=Pt−Pi
Now we substituting the known values we get,
⇒ΔP=1.1−1
On simplifying we get,
ΔP=0.1atm
So, we have calculated that pressure is increased by 0.1atm.
So, 0.1×760mm=76mm for Hg.
The difference in the level of mercury is 76mmHg.
So, the correct answer is Option C.
Note: We have to know that height among the levels of mercury in both columns indicates the pressure difference exerted by the gas in both container and the atmosphere. Likewise, based on the pressure of the system, the levels of the mercury in the nearby tubes increase.
