Question
Question: An octahedral complex of \[\text{C}{{\text{o}}^{+3}}\] is diamagnetic. The hybridisation involved in...
An octahedral complex of Co+3 is diamagnetic. The hybridisation involved in the formation of the complex is:
A)sp3d2
B)dsp2
C)d2sp3
D)dsp3d
Solution
Since, it is given that the complex is diamagnetic, it means all the electrons are paired.
As the coordination complex is diamagnetic i.e. low spin complex then they will be inner orbital complex.
Complete answer:
So in the question it is asked to find the hybridisation of a Co complex and it is given that it is an octahedral complex, hence there are six ligands present and there will be six orbitals involved in the hybridisation.
Now let’s write the Configuration of Co atom which has an atomic number of 27 and thus 27 electrons should be distributed among the orbitals.
The electronic configuration of cobalt is !![!! Ar !!]!! 3d74s2
In the complex the Co is having an oxidation state of +3 .It loses 3 electrons to become Co3+
Electronic configuration of Co3+- !![!! Ar !!]!! 3d6
And it is said that the complex is diamagnetic in nature and that statement passes information that the ligands in the complex are strong ligands.
Since all the electrons pair up only when the ligand is strong. If the ligand is strong then it splits the d-orbitals to a very large extent.
As the d-orbitals are getting split in high magnitude the pairing energy will be less than the Δo and hence the electrons pair up.
Now let’s talk about the hybridisation.
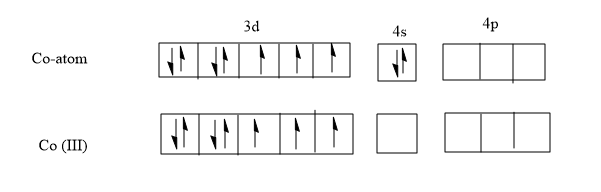
The figure above shows the number of electrons present in each orbitals of Co and Co3+.
So we know that according to the valence bond theory each metal ion makes available, vacant orbitals equal to its coordination number. These vacant orbitals will undergo suitable type of hybridization to give the same number of vacant hybrid orbitals having equivalent energy and definite geometry. The vacant hybrid orbitals of the central metal ion will overlap with the filled orbitals of the ligand to form metal-ligand coordinate bond which is a special type of coordinate bond. The number and type of vacant orbitals taking part in hybridization will decide the type of hybridization and hence the geometry of the molecule.
Here the coordination number is six since the complex is octahedral. Here six hybrid orbitals are formed and the orbitals undergoing hybridisation as the ligands is strong is the two 3d orbital, one 4s orbital and three 4p orbitals and forming six d2sp3hybrid orbitals.
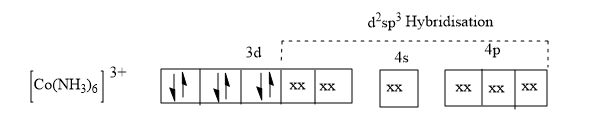
The six hybrids form coordinate bonds with the ligands and form the six coordination bonds.
Note:
The ligand like ammonia is a weak ligand but when it forms complex with Co, it acts as a strong ligand.
Diamagnetic complexes are low spin complexes and hence the d-orbital involved will be inner d-orbital and is called as the inner orbital complexes, since the ligand will be strong.
For paramagnetic complexes, they possess high spin and are called as the high-spin complex or the outer orbital complex, since the d-orbital involved will be outer d-orbitals as the ligands associated will be weak in nature.
