Question
Question: An isomer of ethanol is: A) Methanol B) Dimethyl ether C) Acetone D) Diethyl ether...
An isomer of ethanol is:
A) Methanol
B) Dimethyl ether
C) Acetone
D) Diethyl ether
Solution
Isomers can be defined as compounds with the same chemical formula but different structural formulae. We shall compare each option with the chemical formula of ethanol.
Complete step by step solution:
The chemical formula of ethanol is C2H6O. So let us have a look at the chemical formulae of these compounds given above to determine which one is the isomer of ethanol. This structure is:
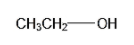
The formula of methanol isCH4O. Hence it can be seen the formula of ethanol doesn’t resemble that of methanol. Hence they are not isomers of each other. The structure is:
CH3−OH
The formula of Dimethyl ether is C2H6O. So it can be seen that both Dimethyl ether and ethanol have the same chemical formula and the same number of atoms. Hence they are isomers of each other. The structure is as follows:
CH3−O−CH3
The formula for acetone is C3H6O. So it can be seen that there is one more carbon atom in the chemical formula than what is there in ethanol. Hence acetone is also not the isomer of ethanol. The structure is given below:
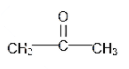
Lastly, the Diethyl ether has formula C4H10O. Here also there is no resemblance of the chemical formula of Diethyl ether with that of ethanol. Hence they are also not the isomers of each other. The structure is given below
CH3CH2−O−CH2CH3
So, the correct answer is option B.
Note:
There are different types of isomers and here the ethanol and dimethyl ether are functional group isomers as their functional groups differ from each other. The other types of isomerism include structural isomerism, positional isomerism, and tautomerism.
