Question
Question: An iso-cyanide on hydrolysis gives A. An amide B. A carboxylic acid and ammonia C. A N-substit...
An iso-cyanide on hydrolysis gives
A. An amide
B. A carboxylic acid and ammonia
C. A N-substituted amide
D. Ethyl amine and formic acid
Solution
We have to know that acid hydrolysis of ethyl iso-cyanide is a nucleophilic replacement reaction, which cuts the triple connection among N and C of iso-cyanide bunch. This happens by protonation of iso-cyanide carbon followed by the assault of water on this electron insufficient carbon molecule.
Complete answer:
Above all else, we will perceive what is acid hydrolysis,
An interaction wherein a protic corrosive is utilized for catalyzing the cleavage of a synthetic bond by the cycle of nucleophilic replacement reaction, alongside addition of components of water is known as acid hydrolysis.
When, the acid hydrolysis likewise alludes to some nucleophilic expansion reaction like the corrosive catalyzed hydrolysis of the nitriles to amides. At the point when we subject any iso-cyanide compound to acid hydrolysis it prompts the development of particular alkyl amine and formic acid. The overall substance condition must be composed as,
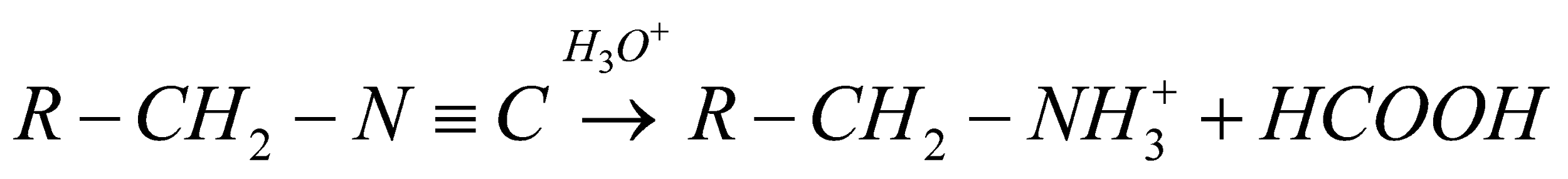
Presently, let us see the sub-atomic equation of ethyl iso-cyanide. It must be given,
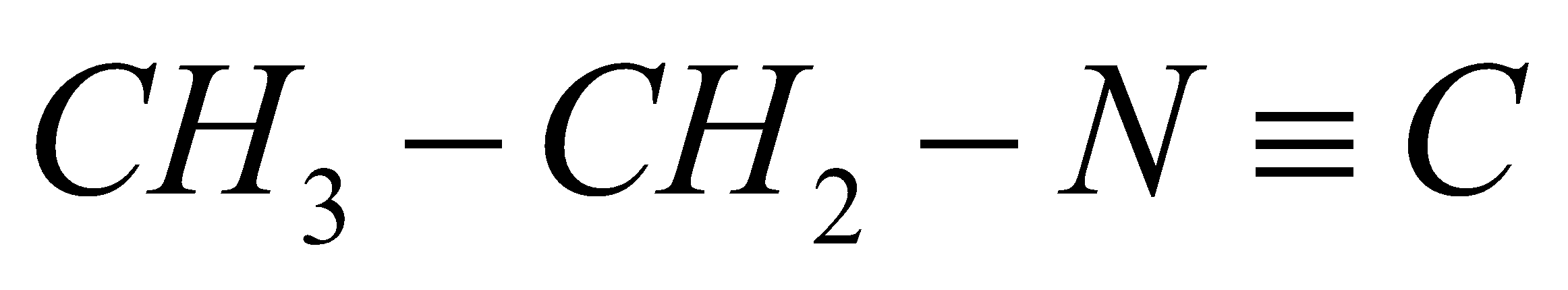 . We as a whole realize that isocyanides are very steady in essential conditions yet they are extremely delicate and receptive under acidic conditions. Here and there a couple isocyanides can additionally polymerize because of the presence of Bronsted and Lewis acids. Consequently, when ethyl iso-cyanide goes through and hydrolysis, the response will continue as,
. We as a whole realize that isocyanides are very steady in essential conditions yet they are extremely delicate and receptive under acidic conditions. Here and there a couple isocyanides can additionally polymerize because of the presence of Bronsted and Lewis acids. Consequently, when ethyl iso-cyanide goes through and hydrolysis, the response will continue as,
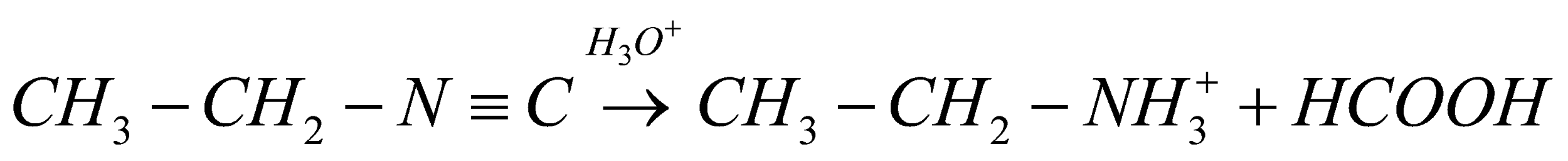
The final products will be ethylamine and formic acid.
Therefore, the correct option is (D).
Note:
We have to know that acid hydrolysis is not the expansion of components of water in a catalyzed way to the twofold or triple bonds through electrophilic expansion as done in the hydration reactions. They are nucleophilic replacement reactions.
