Question
Question: An ion forms the complexes \({[M{({H_2}O)_6}]^{2 + }}\) , \({[M{(en)_3}]^{2 + }}\) and \({[MB{r_6}]^...
An ion forms the complexes [M(H2O)6]2+ , [M(en)3]2+ and [MBr6]4− . Match the complex with appropriate color:
A.Green, blue and red
B.Blue, red and green
C.Green, red and blue
D.Red, blue and green
Solution
We should know the order of ligand field strength (spectrochemical series) and also the energies for the colors. Every color has a specific wavelength and energy. Each ligand has a different energy of transition and this energy arrange In a reliable way.
Complete step by step answer:
All the given complexes are octahedral. Mostly the coordination compounds are colored due to the electronic transition between the energy levels. The intensity of absorption of light depends on the strength of ligands too.
The strength of the ligands is given by a series known as the spectrochemical series. In this series ligands are arranged according to the increasing energy of transition that occur when these ligands are present in a complex. The series is as follows-
I−<Br−<S2−<SCN−<Cl−<F−<OH−<C2O42−<O2−<H2O<NCS−<NH3<en<NO2−<CN−<CO
In this series CO is the ligand that will give rise to high energy transition in the complex. Such ligands are called strong field ligands. As we move from left to right in spectrochemical series, the energy of transition increases. For example, for the same metal the absorption of the cyano (CN−) complex will occur at higher energy than that of the corresponding chlorido (Cl−) complex.
In the given example, the metal atom is same and ligands are different in each case. Now if we observe the ligands and the spectrochemical series, the order of strength of ligands can be given as-
Br−<H2O<en
The order for the energies of the color is-
violet>blue>green>yellow>orange>red
Now let us talk about the absorption of light. While explaining the colors of co-ordination compounds we have to deal with the phenomenon of complimentary colors. It means that when a compound absorbs light of one color, we see the compliment of that color. For example when white light passes through a substance and that substance absorbs red light from the spectrum, the color observed is green. Hence green is compliment of red. Green predominates visually when red light is subtracted from the white light. Color wheel is used to understand the concept of complementary colors. The opposite pair on the color wheel are complement of one another.
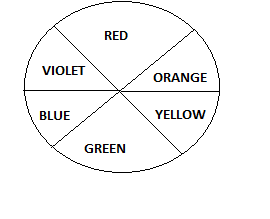
Now as we know [M(en)3] has the highest absorption. It will absorb green and appear as red.
[M(H2O)6] has intermediate energy absorption among the three. It will absorb orange and appear blue.
[M(Br)6] has lowest absorption among all. It will absorb red and appear green.
So, the correct option is B.
Additional information:
The two main geometries of coordination compounds are tetrahedral and octahedral geometry. In tetrahedral geometry metal is attached to four ligands whereas in octahedral metal atoms are attached to six ligands.
Note:
Remember energy and wavelength have opposite relations. For example, red has the lowest energy so it has the highest wavelength. Similarly if a complex has high energy transition then the wavelength of that transition will be low. The unit commonly used for measuring wavelength is nanometer (nm) .
