Question
Question: An insulating cylinder contains equal volumes of He and O₂ separated by a massless freely moving adi...
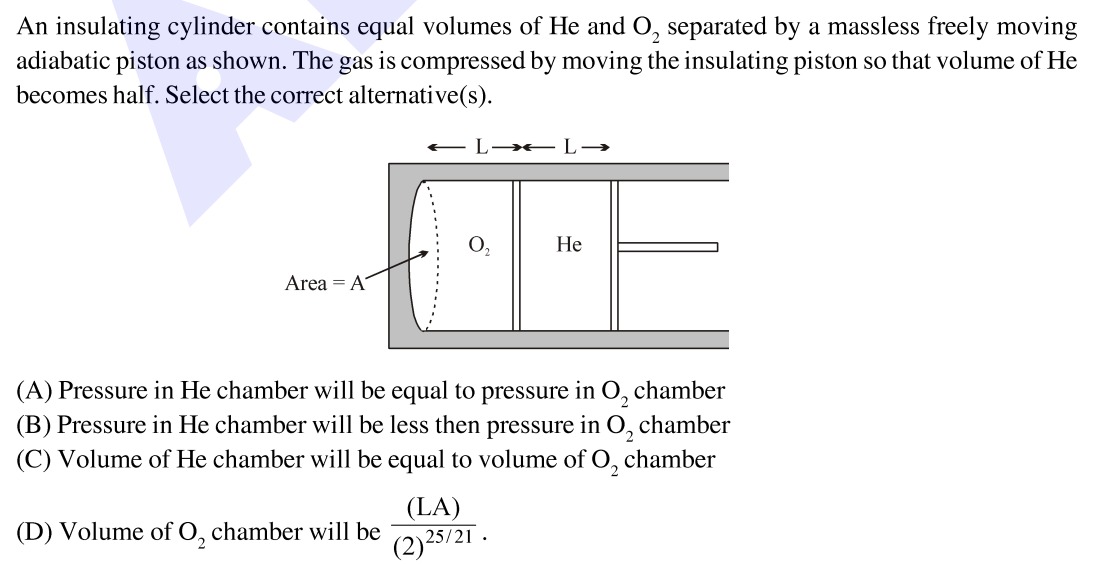
An insulating cylinder contains equal volumes of He and O₂ separated by a massless freely moving adiabatic piston as shown. The gas is compressed by moving the insulating piston so that volume of He becomes half. Select the correct alternative(s).
Pressure in He chamber will be equal to pressure in O₂ chamber
Pressure in He chamber will be less then pressure in O₂ chamber
Volume of He chamber will be equal to volume of O₂ chamber
Volume of O₂ chamber will be (2)25/21(LA)
A, D
Solution
Let the initial volume of He and O₂ be V0. From the diagram, if the area of the piston is A and the initial length of each chamber is L, then V0=LA.
Initially, the piston separating He and O₂ is massless and freely moving, so the pressure in both chambers is equal: PHe,1=PO2,1=P1.
The gas is compressed adiabatically such that the final volume of He is VHe,2=21VHe,1=2V0.
Since the piston separating He and O₂ is adiabatic and freely moving, the pressure in both chambers in the final state is also equal: PHe,2=PO2,2=P2.
The compression process for each gas is adiabatic.
For He (monatomic gas, γHe=5/3):
PHe,1VHe,1γHe=PHe,2VHe,2γHe
P1V05/3=P2(2V0)5/3
P2=P1(V0/2V0)5/3=P1(2)5/3.
For O₂ (diatomic gas, γO2=7/5):
PO2,1VO2,1γO2=PO2,2VO2,2γO2
P1V07/5=P2VO2,27/5
Substituting P2=P1(2)5/3:
P1V07/5=P1(2)5/3VO2,27/5
V07/5=(2)5/3VO2,27/5
VO2,27/5V07/5=25/3
(VO2,2V0)7/5=25/3
VO2,2V0=(25/3)5/7=2(5/3)×(5/7)=225/21.
VO2,2=225/21V0.
Now let's check the options:
(A) Pressure in He chamber will be equal to pressure in O₂ chamber. This is correct because the separating piston is massless and freely moving.
(B) Pressure in He chamber will be less than pressure in O₂ chamber. This is incorrect.
(C) Volume of He chamber will be equal to volume of O₂ chamber. VHe,2=V0/2. VO2,2=V0/225/21. Since 25/21>1, 225/21>2. Thus 1/225/21<1/2. So VO2,2<VHe,2. This is incorrect.
(D) Volume of O₂ chamber will be (2)25/21(LA). Since V0=LA, we have VO2,2=225/21LA. This is correct.
