Question
Question: An ideal monoatomic gas is confined in a horizontal cylinder by a spring-loaded piston. Initially, t...
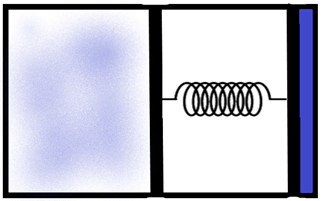
An ideal monoatomic gas is confined in a horizontal cylinder by a spring-loaded piston. Initially, the gas is at temperature T1, pressure P1 and volume V1 and the spring is in its relaxed state. The gas is then heated very slowly to temperature T2, pressure P2 and volume V2. During this process the piston moves out by a distance x. Ignoring the friction between the piston and the cylinder, the correct statement/s is/are –
A) If V2=2V1 and T2=3T1, then the energy store in the spring is 41P1V1
B) If V2=2V1 and T2=3T1, then the change in internal energy is 3P1V1
C) If V2=3V1 and T2=4T1, then the work done by the gas is 37P1V1
D) If V2=3V1 and T2=4T1, then the heat supplied to the gas is 617P1V1.
Solution
We need to understand the mechanisms involved and the relation between the thermodynamic variables – the pressure, volume and temperature – with the energy in the system or work done by the system as required in the problem.
Complete step by step solution:
We are given a piston which is built with a spring loaded to it as shown in the figure. We know that for a system of ideals, when the temperature increases, the pressure inside it increases, which in this case causes the piston to be pressed causing a subsequent increase in the volume also.
Now, let us consider the piston. The total area of the piston base where the gas molecules exert the pressure is ‘A’. We can calculate the total pressure acting on the piston as the initial pressure in the system and the force due to the spring of spring constant ‘k’ as –
