Question
Question: An ideal monatomic gas is taken around the cycle \(ABCDA\) as shown in \(P - V\) diagram. The Work d...
An ideal monatomic gas is taken around the cycle ABCDA as shown in P−V diagram. The Work done during the cycle is
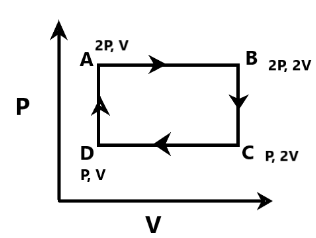
A. PV
B. 0.5PV
C. 2PV
D. 3PV
Solution
The work done by an ideal gas can be calculated using the P−V diagram or the curve from the specific graph. Based on the plot the work done can be calculated as the pressure and volume at different time points for the given ideal gas can be calculated from the given graph itself.
Complete step-by-step answer: The given P−V diagram or plot has the cyclic process given with the ABCDA plot. The work done needs to be calculated using the area of the cyclic process that is given in the plot as ABCDA. The whole cyclic process can be presented as a rectangular structure which is why the area of the rectangular structure ABCDA can be considered as the work done by the monatomic gas in the specific time point. From the given rectangular cyclic structure, the area can be calculated as BA×DA. The BA can be calculated as the difference of the pressure and volume from the point B to point A. There is a volume change observed, which is why the value of BA in the given cyclic process is V. The DA can be calculated based on the differences between pressure and volume. There is a change observed only in the pressure between the time points D to A. This is why the value of DA in the cyclic process can be defined as P. Therefore, the work done in the cyclic process ABCDA can be calculated using the formula BA×DA. This is why from the values the work is done will be: W=P×V. Therefore, the work done in the given process for this cyclic process will be PV.
Hence the correct option is (A).
Note: The P−V diagram is the graphical representation of the changes in the physical property of the gases which shows the changes in the pressure and volume with respect to time. The changes in the physical properties can define the condition in which the monatomic gas is present.
