Question
Question: An ideal gas undergoes a thermodynamic process described by the equation: $PV^2 = C$, where C is a c...
An ideal gas undergoes a thermodynamic process described by the equation: PV2=C, where C is a constant. The gas transitions from an initial state (P1,V1,T1) to a final state (P2,V2,T2). Which of the following statements is correct?
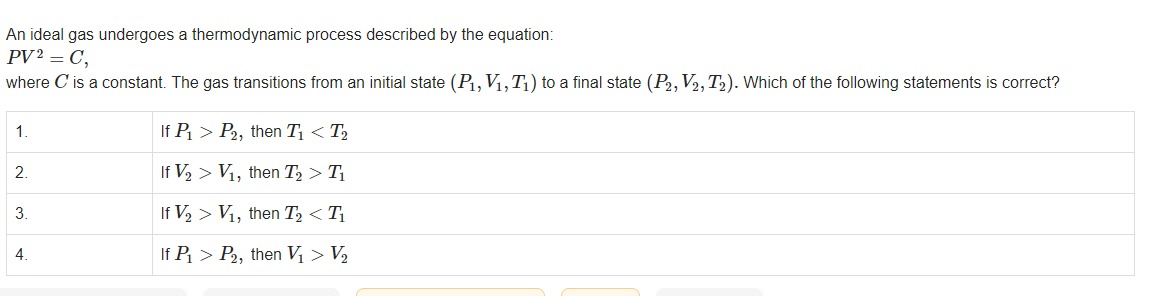
If P1>P2, then T1<T2
If V2>V1, then T2>T1
If V2>V1, then T2<T1
If P1>P2, then V1>V2
If V2>V1, then T2<T1
Solution
The ideal gas law is PV=nRT. The given process is PV2=C.
From these, we can express temperature T as T=nRPV.
-
Substituting P=C/V2 into the expression for T: T=nR(C/V2)V=nRVC. This shows T∝V1.
-
Substituting V=C/P into the expression for T: T=nRPC/P=nRCP. This shows T∝P.
Option 3 states: If V2>V1, then T2<T1. This is consistent with the relationship T∝V1, as an increase in volume implies a decrease in temperature for this process.
