Question
Question: An ideal gas is taken through the cycle\(A\to B\to C\to A\),\(\Delta U\) as shown in the figure. If ...
An ideal gas is taken through the cycleA→B→C→A,ΔU as shown in the figure. If net heat supplied to the gas in the cycle is5J, the work done by the gas in the processC→A is:
(A) +5J
(B) +10J
(C) +15J
(D) +20J
Solution
By using the first law of thermodynamics find work done where net heat is 5J. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. In equation form, the first law of thermodynamics: ΔU=q+W.
Complete step by step answer:
The first law of thermodynamics is based on experience that energy can be neither created nor destroyed, if both the system and the surrounding are taken in account we can conclude it by saying energy is conserved.
Direct consequence of this statement is U that is a state function. This implies that between any two fixed states ,there can be infinite processes or paths but ΔU’ in all processes will remain the same U
Here as we know that the net heat applied in this process is5J.
Now returning to the initial point which is A, as we know Internal energy is a state function, which means that A is a constant before and after the cyclic process.
Now according to first law of Thermodynamic
ΔU=q+W
Here, ΔU= Change in internal energy
q=heat added
W= work done
So we can say that
ΔU=0
q=−W OR W=−q
Now we can say that
WA→C=WA→B+WB→C−q ……….(1)
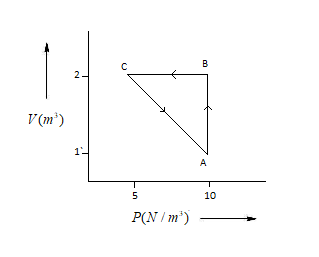
Here, WA→C=Work done in A to C
WA→B=Work done in A to B
WB→C= Work done in B to C
WA→B=PΔV=10(2−1)=10.............(2)
Here, P=Pressure (Unit=N/m3)
ΔV= Volume (Unit=m3)
WB→C=PΔV=0 .................(3)
(∵ ΔV=0)
By putting value of equation (2) and (3) in equation (1) we get
WA→C=10+0−5
∴ The work done by the gas in the process C→A is:
WA→C=5J
Hence the correct option is (A)+5J.
-Since the temperature of the gas changes with its internal energy, it follows that adiabatic compression of a gas will cause it to warm up, while adiabatic expansion will result in cooling.
Note:
-If there is no change in temperature it means internal energy is Zero.
-In Adiabatic change the q=0 due to which the first law of thermodynamics is ΔU=0+W.
-Observe the graph carefully to find the change in volume.
