Question
Question: An ideal gas is taken through a cycle ABCA as shown in the figure. The work done during the cycle is...
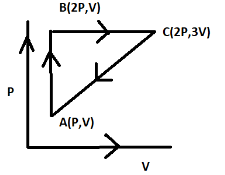
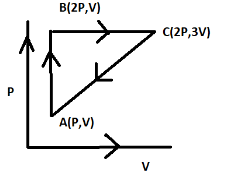
An ideal gas is taken through a cycle ABCA as shown in the figure. The work done during the cycle is:
Solution
A gas whose molecules occupy negligible or no space at all and have no interaction within the molecules are known as ideal gas. And also the gas which consequently obeys the gas laws is exactly defined as the ideal gas. This ideal gas refers to the hypothetical gas which is composed of molecules and these molecules should obey certain rules. The molecules in the ideal gas do not attract or repel each other. The molecules of ideal gas themselves take up no volume.
Complete Step By Step Answer:
Work done in thermodynamics is defined as the energy it takes to move an object against a force. The system’s internal energy decreases when a system does work.
Now from the given diagram, we can find the work done by calculating the area under the graph or triangle.
Area of the triangle =21b×h
Here, b is the breadth of the triangle and h is the height of the triangle.
The breadth of the triangle is found to be b=3V−V . (Since the x-axis refers to the volume we only take the volume points)
The height of the triangle can be calculated to be h=2P−P . (Since the y-axis refers to the pressure we only take the pressure points)
Substituting this in the formula we get,
⇒21×(3V−V)×(2P−P)
⇒21×2V×P
On simplifying the above equation we get,
⇒PV
Therefore the work done by the ideal gas in the given graph is PV .
Note:
We should not get confused while calculating the area under the graph. We shouldn’t be subtracting V from 3V because that is going from B to C. Though it is going in that direction we cannot subtract in that way because it would yield a negative answer and area is a real quantity therefore it cannot be negative.
