Question
Question: An ideal gas is initially at temperature \(T \) and volume \(V\). Its volume is increased by \(\Delt...
An ideal gas is initially at temperature T and volume V. Its volume is increased by ΔV due to an increase in temperatureΔT, pressure remaining constant. The quantity δ=VΔTΔV varies with temperature as:
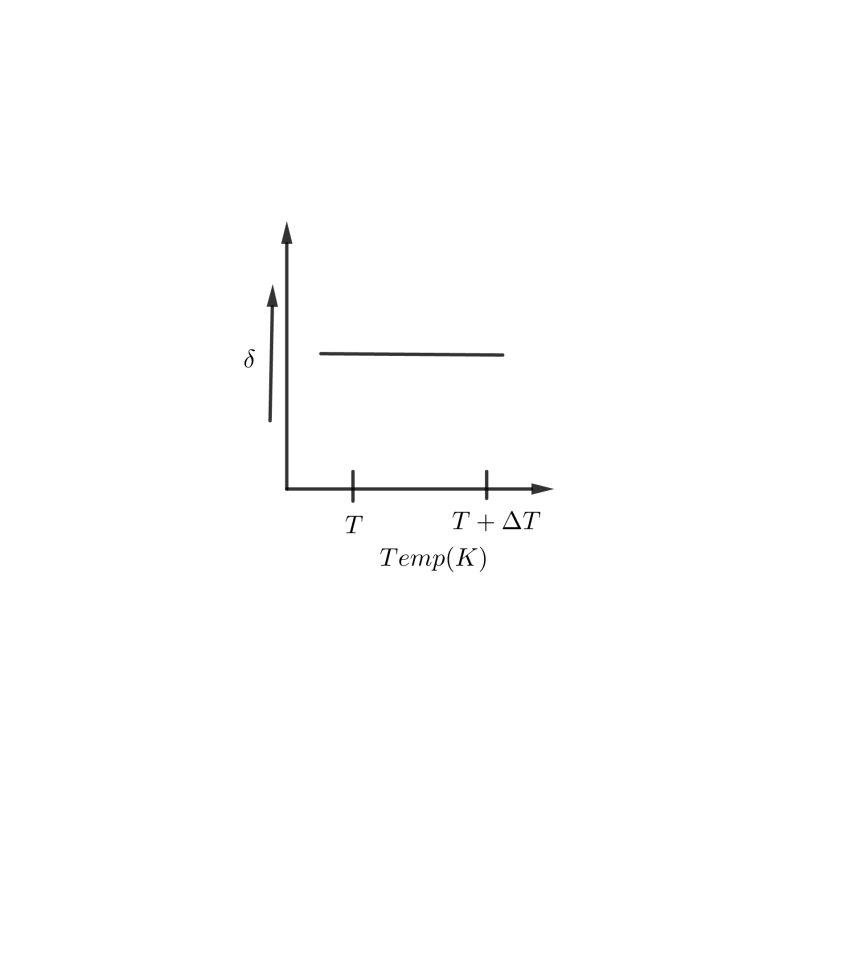
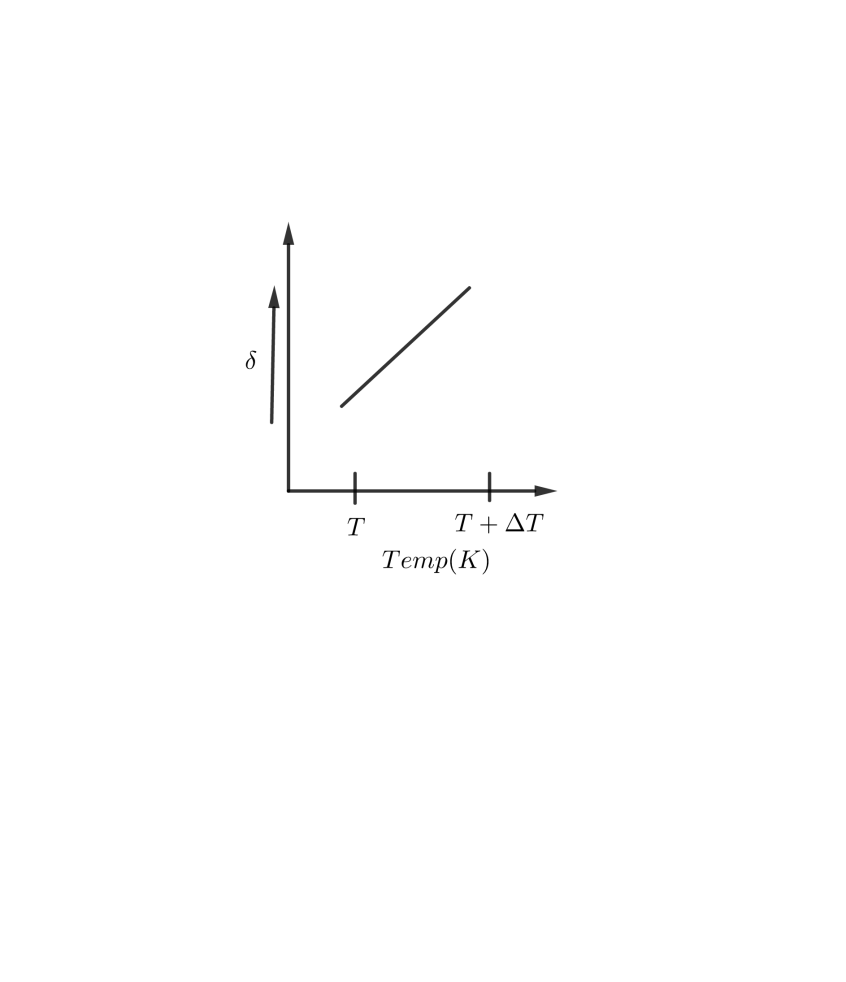
A B
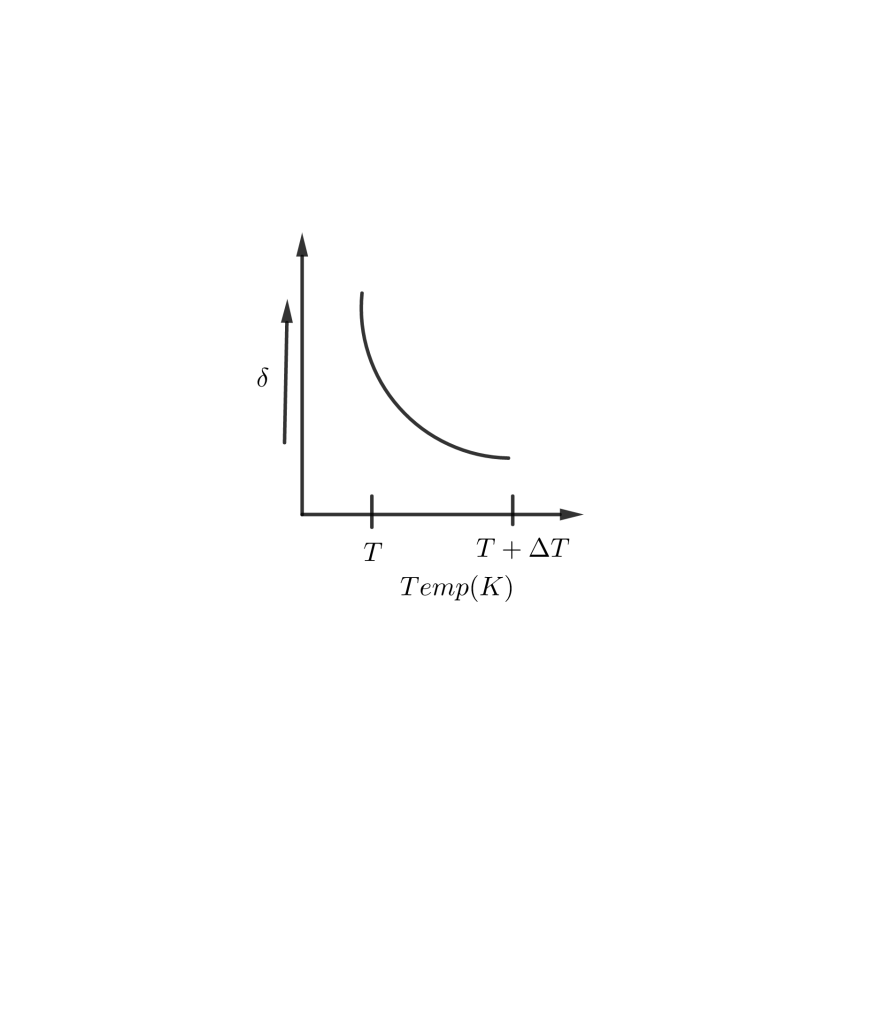 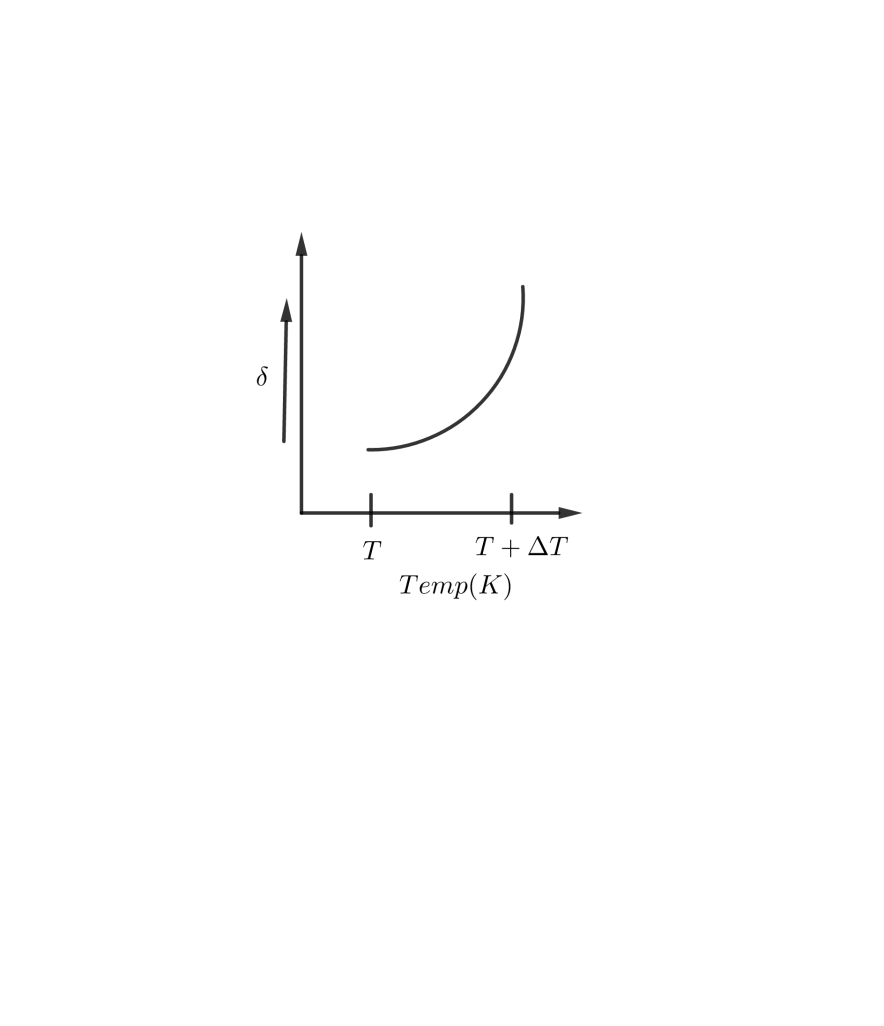
C D
Solution
Use ideal gas law to find the relation between volume and temperature. Differentiate it to find the relation between the error in volume and the error in temperature.
PV=nRT
Here,
P is pressure V is volumen is number of molesR is ideal gas constantT is temperature
Complete step by step answer:
From the ideal gas law,
TV=PnR
As n and R are constants,
TV=constant at constant pressure
Differentiate with respect to T keeping V constant, and then differentiate with respect to V keeping T constant,
TΔV+−T2VΔT=0
⟹VΔTΔV=T1
⟹δ=T1
Thus, when T increases, δ decreases and vice-versa.
So, the correct answer is “Option C”.
Additional Information:
δ is known as the volumetric thermal expansion coefficient.
A process in which the pressure remains constant is known as an isobaric process. The heat which is transferred to the system does the work and changes the internal energy of the system
Note:
TV=constant, can be stated as: volume of an ideal gas at constant pressure is directly proportional to its temperature. This is known as Charles’s law. This law combined with Boyle’s law, Avogadro’s hypothesis and Gay-Lussac’s law gives the equation of state for an ideal gas, the ideal gas law.
As T tends to low values, δ increases rapidly. At large values of T, δ tends to low values and becomes almost constant.
