Question
Question: An ideal binary solution is prepared by two liquids A and B, with \[P_A^0 > P_B^0\] . Then: A. A-...
An ideal binary solution is prepared by two liquids A and B, with PA0>PB0 . Then:
A. A-B interactions are stronger than A-A and B-B
B. A and B have the same molecular masses
C. A-B interactions are similar to A-A and B-B interactions
D. Both A and B are non-polar substances
Solution
For an ideal gas, the enthalpy of the solution gets closer to zero and shows an ideal behavior. Also, the volume remains the same in mixing the solution. For vapor pressure, one must know Raoult’s law. The entropy always increases for an ideal gas and so the Gibbs free energy is negative.
Complete step by step answer:
Raoult’s law states that a solvent’s partial vapor pressure in a mixture or solution is identical to the vapor pressure of the pure solvent multiplied by its mole fraction. Solutions that obey this law are ideal and which do not obey are non-ideal solutions.
For an ideal solution, as per Raoult’s law, the partial vapor pressure of a solvent in a solution is equal to the vapor pressure of a pure solvent multiplied by its mole fraction.
We have PA=xAPAo,PB=xBPBo
Therefore, ΔP=Pobs−PcalculatedbyRaoult′slaw=0
Therefore, an ideal binary solution is prepared by two liquids A and B, with PA0>PB0 . Then A-B interactions are similar to A-A and B-B interactions.
So, the correct answer is C.
Additional information:
An ideal solution is a homogeneous mixture of compounds under which the interactions between the solute and solvent molecules are the same as those between the molecules itself of each substance. For example, benzene and toluene have very similar molecular structures and so they are ideal.
We know that, ΔUmix=ΔHmix−PΔVmix from the first law of thermodynamics
For an ideal solution, the solution enthalpy reached approximately to zero and the volume mixing is also zero for an ideal gas. During mixing, no heat should be either evolved or absorbed and there should be no expansion or contraction during mixing.
ΔHmix=0,ΔVmix=0
∴ΔUmix=0
For ideal solution, the Gibbs free energy can be written as:
ΔGmix=ΔHmix−TΔSmix
ΔSmix=0 for an ideal gas
Thus, ΔGmix=0
Note: The solutions which do not obey Raoult’s law can be volatile or non-volatile. They can either dissociate or associate in a solution. Because the solutions that obey the law have these limitations that the solution should contain non-volatile solutes only and must not associate or dissociate in solution.
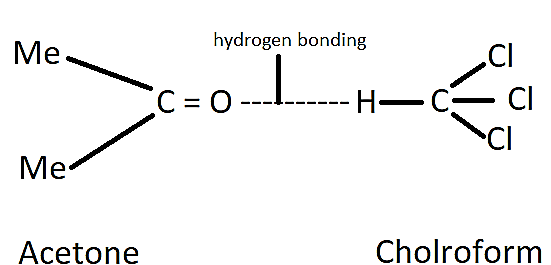
For example, chloroform and acetone show a negative deviation from Raoult’s law. This is because there is an attractive interaction between these two that results in the formation of a hydrogen bonding as shown below.
__