Question
Question: An ice cube is floating in water above which a layer of lighter oil is poured. As the ice melts comp...
An ice cube is floating in water above which a layer of lighter oil is poured. As the ice melts completely, the level of water surface (or interface) and upper level of oil will
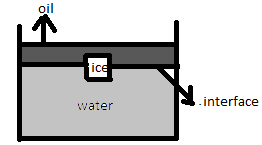
A. Rise and fall
B. Fall and rise
C. not change and change
D. not change and fall not
Solution
Here we have to apply the concept of density mass and volume-
The mass per unit volume is the density of a material.
Density can be mathematically written as-
Density=volumemass
Complete step by step solution:
In the question it is given that-
An ice cube is floating in water above which a layer of lighter oil is poured.
We have to find out what will happen to the level of water and oil if the ice melts completely-
Since, before and after melting, the amount of the ice will remain same-
So, Mass = Density × Volume ...... (1)
Case 1: As the water density is greater than that of ice-
So, we can say using equation (1) that after melting the portion of ice that is within the water, more water volume will be created than the volume of the corresponding ice and the water level will be increased.
Case 2: As the light oil density is smaller than that of ice-
Since light oil has a lower density than ice, we can also say by equation (1) that the portion of ice within oil contains less water than its actual volume. This would reduce the oil volume.
So, from the above discussion we can conclude that as the ice melts completely, the level of water will increase and the level of oil will decrease.
**Hence, option A is correct.
Note:**
Here since ice also gets converted to water on melting so it will merge with the water already present and thus the water interface will increase. But oil is completely different from water and after the ice melts it does not mix with the oil. So, the level of oil will decrease.
