Question
Question: An exothermic reaction proceeds by two stages: \[\operatorname{Reactant}\xrightarrow{Stage\,1}Inte...
An exothermic reaction proceeds by two stages:
ReactantStage1IntermediateStage2Products
The activation energy of state 1 is 50kJmol−1. The overall enthalpy change for the reaction is −100kJmol−1. Which diagram could represent the energy level diagram for the reaction?
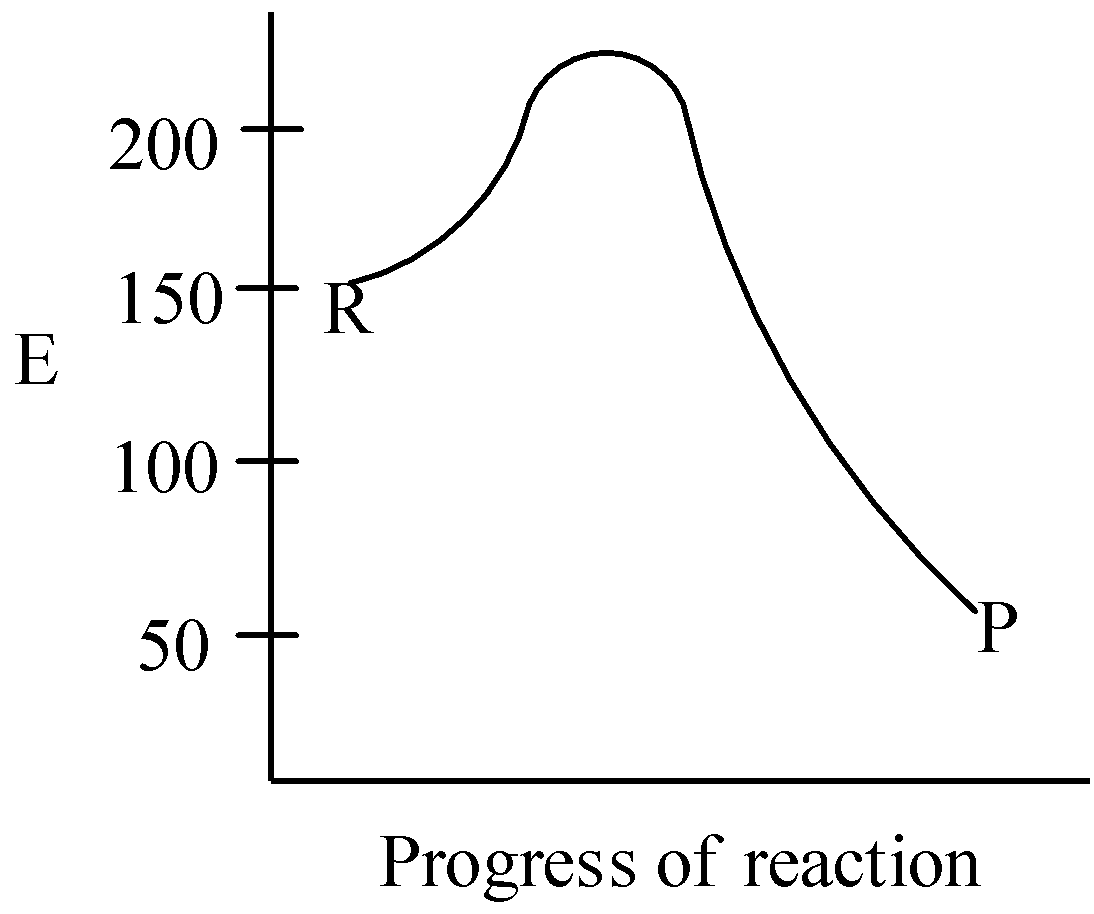
A.
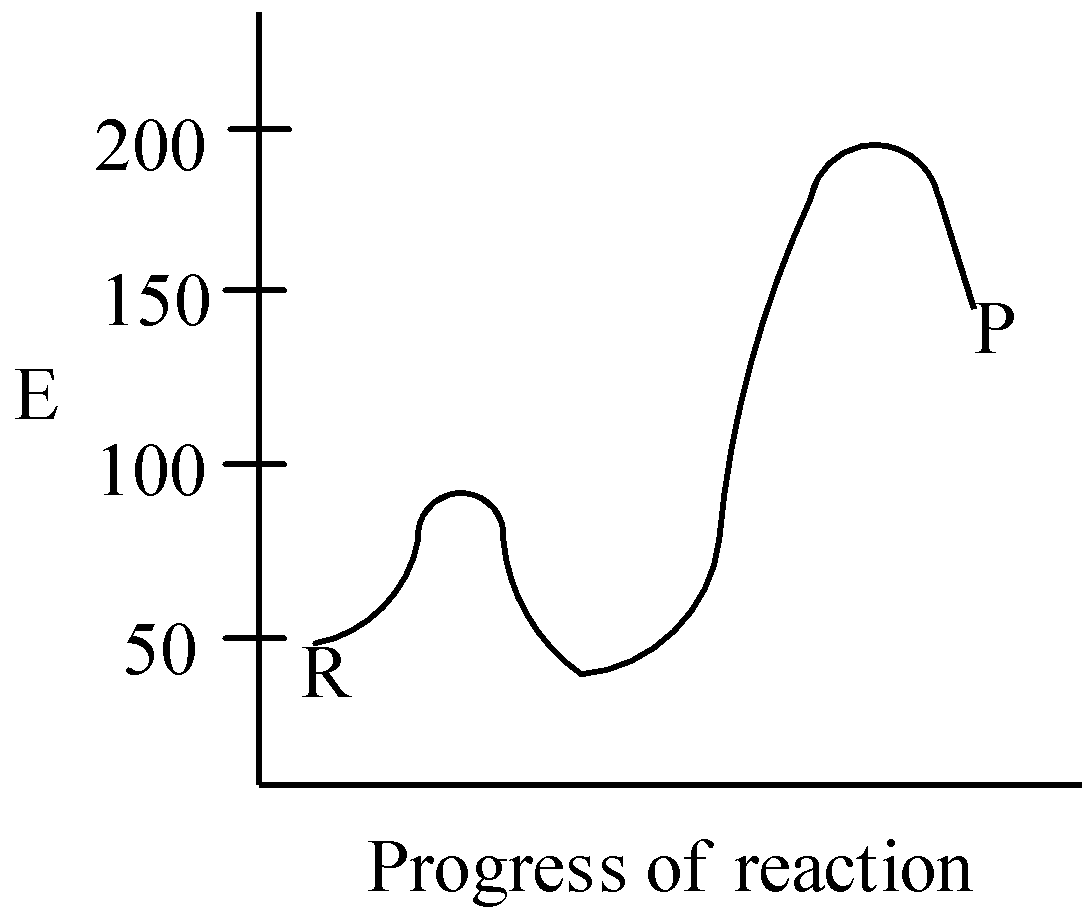
B.
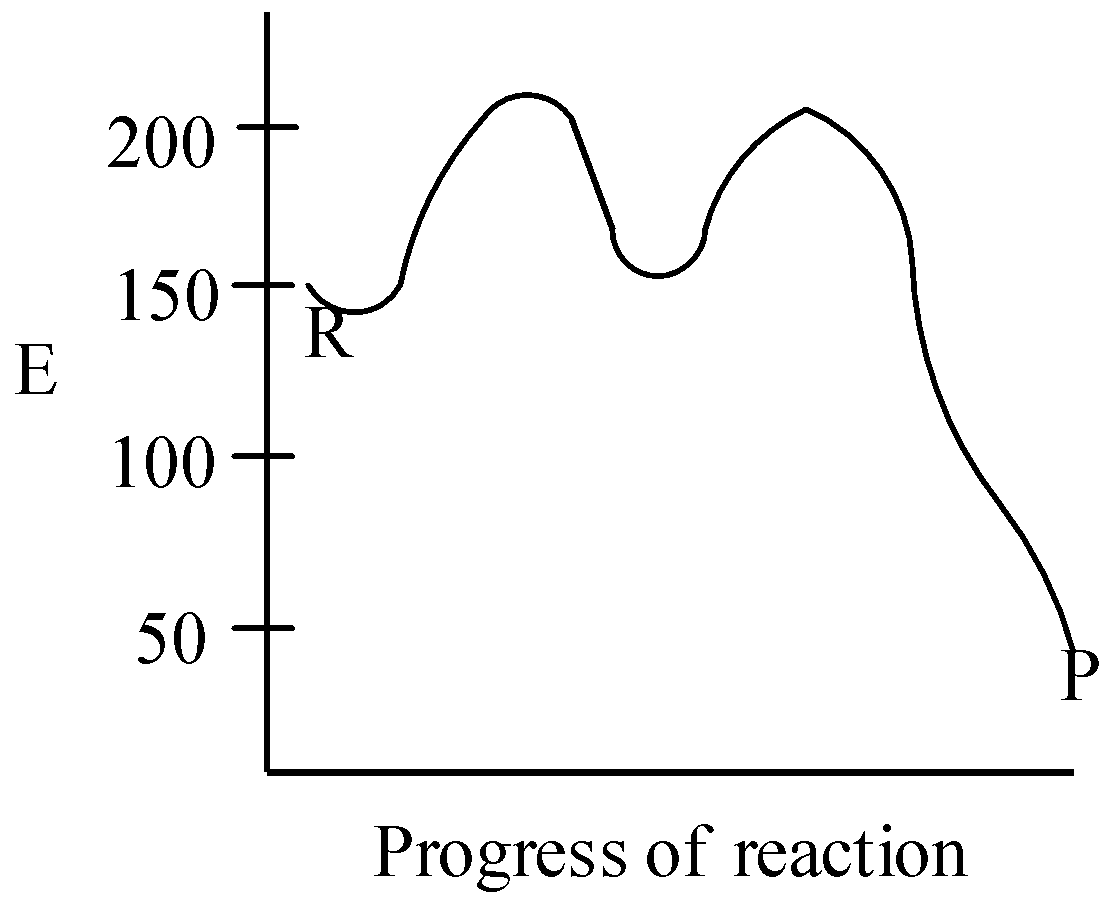
C.
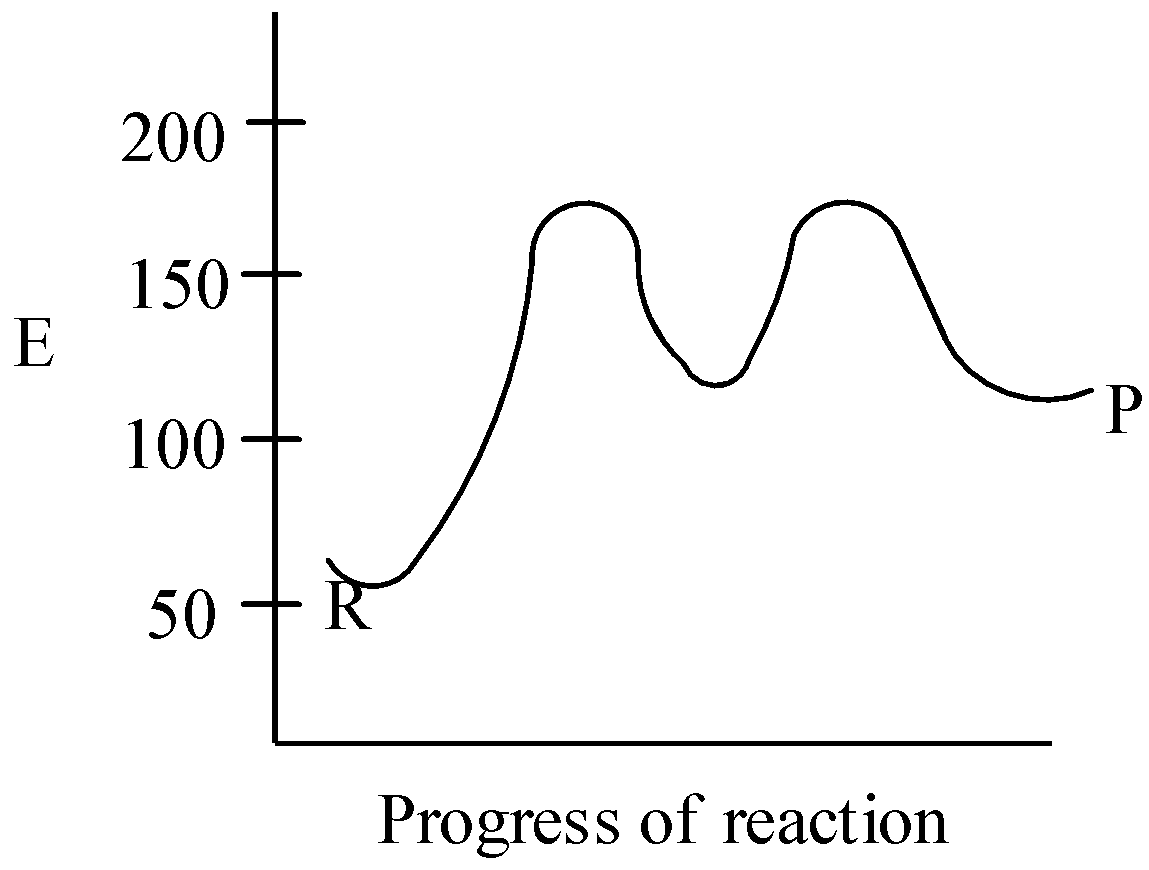
D.
Solution
In an exothermic reaction, ΔH=−ve, as heat is given out in surroundings, and in endothermic reaction, ΔH=+ve, as heat is absorbed from the surroundings.
Complete answer:
In order to answer our question, we need to know about chemical kinetics and exothermic and endothermic reactions. We know that every reaction has some activation energy. This activation energy helps in breaking the intermolecular bonds and helps in combination of different elements. Enthalpy can be considered as the internal heat or internal energy change, during a reaction. This enthalpy change can be positive as well as negative. Exothermic reactions are those reactions, in which heat is released. In other words, they emit out energy in the form of heat. Endothermic reactions, on the other hand, require heat from the surroundings, and so, environment becomes cooler after the reaction has taken place.
Now it is to be noted that the internal enthalpy change in case of exothermic reactions is negative, whereas in case of endothermic reaction, it is positive. It is so because in an endothermic reaction, the heat is gained, and in an exothermic reaction, heat is lost. With this concept let us solve the question.
The activation energy for the reaction is given to be 50kJmol−1, and there are two stages of the reaction. As the overall enthalpy change of the reaction ΔH=−100kJmol−1, so the reaction is an exothermic reaction. This means the graph should have the lowest value of “E”. Clearly, we can see that in graph C, as progress of reaction increases, the energy decreases, giving out heat to surroundings.
This means that option C is the correct answer for our question.
Note:
It is to be noted that enthalpy is always counted as enthalpy change. The difference is taken because it is impossible to practically reach zero and sub zero temperatures.
