Question
Question: An excited \(H{e^ + }\) ion emits two photons in succession, with wavelengths 108.5 nm and 30.4 nm, ...
An excited He+ ion emits two photons in succession, with wavelengths 108.5 nm and 30.4 nm, in making a transition to ground state. The quantum number n, corresponding to its initial excited state is (for photon of wavelength λ, energy E=λ(in nm)1240eV)
A. n = 5
B. n = 4
C. n = 6
D. n = 7
Solution
The total energy emitted by the two de-excitations is equal to the sum of the energy of the two de-excitations and the Bohr model gives expression for total energy emitted when a hydrogen-like atom undergoes de-excitation from a higher energy state to lower energy state. By equating this formula and the sum of the given energies, we can find out the required value of n for the excited state.
Formula used:
According to Bohr model, the expression for total energy emitted when a hydrogen-like atom undergoes de-excitation from a higher energy state to lower energy state is given by the following formula.
E=13.6Z2[n121−n221]eV
The energy is given in terms of the wavelength of a light by the following expression.
E=λhc
Complete step by step answer:
We are given an excited He+ ion which is a hydrogen-like atom. It emits two photons in succession while making a transition to the ground state from the excited state. The two wavelengths emitted are given as
λ1=108.5nm λ2=30.4nm
The following diagram shows the energy levels for the given atom where the de-excitation takes from an energy level n to the ground state n = 1
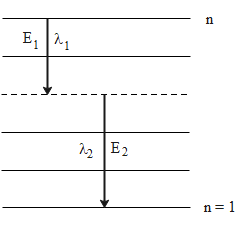
The energy emitted by a hydrogen-like atom in a single de-excitation from an excited state n2 to a lower energy state n1 is given by the following formula.
E=13.6Z2[n121−n221]eV
Now for the given atom, Z = 2 and the total energy emitted by the two de-excitations is equal to the sum of the energy of the two de-excitations. Therefore, we have
E=E1+E2 ⇒13.6Z2[121−n21]=λ1hc+λ2hc ⇒13.6×(2)2[121−n21]=108.5nm1240eV/nm+30.4nm1240eV/nm=11.43+40.79=52.22 ⇒1−n21=54.452.22 ⇒n21=1−54.452.22=54.42.18 ⇒n2=2.1854.4=25 ∴n=5
This is the required value of n for the excited state. Hence, the de-excitation takes from n = 5 state, i.e. the fourth excited state,
So, the correct answer is option A.
Note:
Hydrogen-like atoms are those which have the same electronic configuration as that of a neutral hydrogen atom. The neutral helium atom is not hydrogen-like but if we remove one electron from it then we get the same electronic configuration as hydrogen. Similarly, other hydrogen-like atoms are Li2+, Be3+, etc.
