Question
Question: An example of pyrosilicate is: A) Thortveitite B) Willemite C) Spodumene D) Kaolinite...
An example of pyrosilicate is:
A) Thortveitite
B) Willemite
C) Spodumene
D) Kaolinite
Solution
The silicate is formed by fusing an alkali with the SiO2. It exists in various allotropes like Orthosilicate, pyrosilicate, cyclic silicate, chain silicate, etc. The pyrosilicate consists of the Si2O76- anions which are joined together by the removal of oxygen. The thortveitite is an example of silicate, it is scandium yttrium silicate. (Sc,Y)2Si2O7
Complete step by step answer:
The SiO2 fuses with the alkali oxides to give silicates. This is discrete tetrahedral units. Since silicon is sp3 hybridized. The various units are linked in several patterns and classified as follows,
- Orthosilicate: Orthosilicate contains the separated SiO4units. This is linked together by various patterns. An example is Willemite (ZrSiO4).
- Pyrosilicate: In pyrosilicate, the two units are linked together by the oxygen atom. The simplest ion is Si2O7 . Example is Thortveitite Sc2[Si2O7]
- Cyclic silicates: this allotrope shares the two oxygen atoms. The example is Beryl - Be3Al2Si6O18
- Chain silicates: these are formed by the linking of units to form a chain silicate. They are of two types:
i) Metasilicates
ii) Amphiboles
Now, we will discuss pyrosilicate. A pyrosilicate is a chemical compound that contains the phyllosilicate anion Si2O76- with the hexavalent group −(O3Si-O-SiO3)− .
Pyrosilicate is formed by joining the two tetrahedral units Si2O44−.this units joined together by the removal of oxygen and two units then join at the corners of oxygen atoms.
The structure of pyrosilicate is as follows:
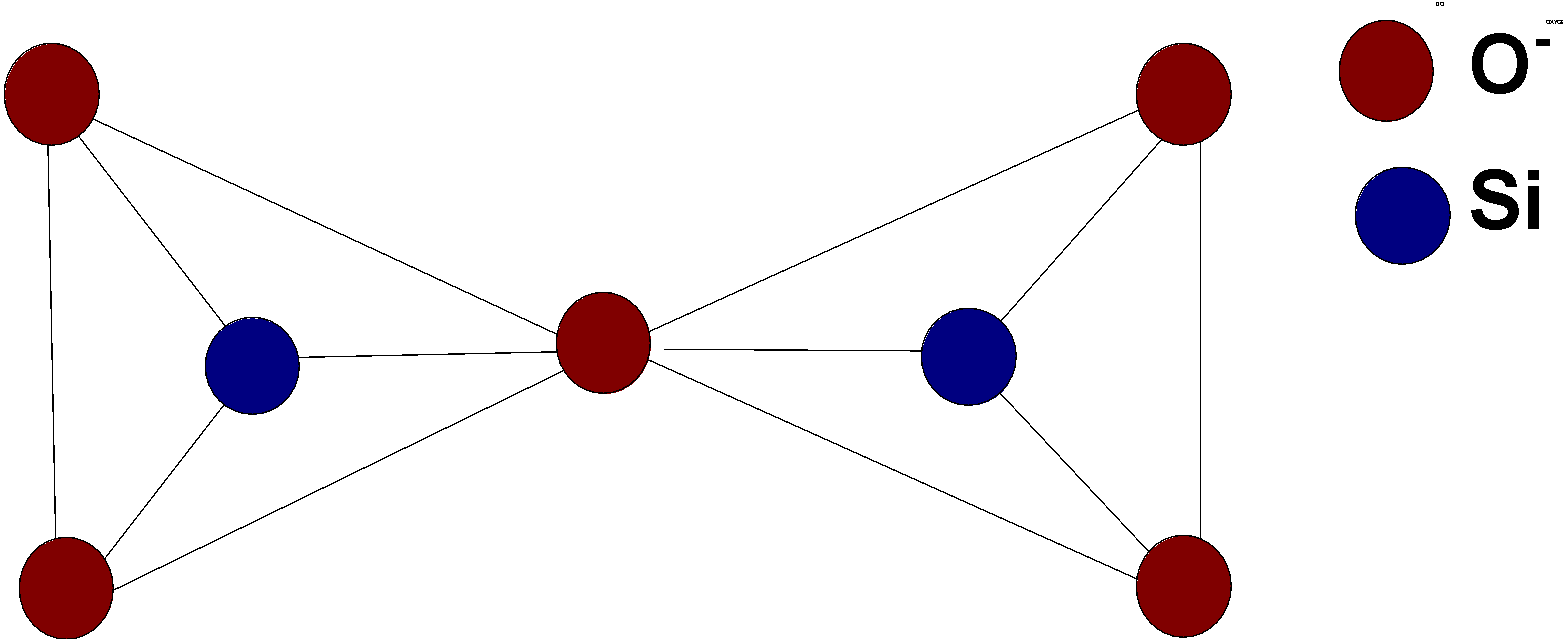
One of the common examples of pyrosilicate is thortveitite. It is a scandium silicate yttrium silicate (Sc,Y)2Si2O7. It is a primary source of scandium.
Hence, (A) is the correct option.
Note: The silicon is a group 14 element and shows the allotropic properties. The
silicates have the silicon-oxide linkage but exist in a different structure. Students should remember the formulas for the silicates.
