Question
Question: An equimolar-quantities of ethanol and propanol is heated with conc. \({{H}_{2}}S{{O}_{4}}\). The pr...
An equimolar-quantities of ethanol and propanol is heated with conc. H2SO4. The product formed is/are?
(A) C2H5OC2H5
(B) C3H7OC3H7
(C) C2H5OC3H7
(D) All of these
Solution
As two types of alcohol are involved in the reaction, formation of only a single product is impossible.
Complete step by step solution:
As, we can see that the equations are all ethers. Thus, formation of ether is involved when alcohol is heated with conc. H2SO4.
Etherification-
Alcohols undergo dehydration to produce alkenes and ethers (at some special conditions) in the presence of H2SO4.Formation of ether from the dehydration of alcohol is a nucleophilic substitution reaction. Generally,
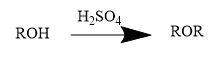
Thus,
When alcohol is heated with conc. Sulphuric acid, the reaction follows two steps of mechanism.
1. Formation of alkyl hydrogen sulphate.
2. Formation of dialkyl ether.
The large amount of alcohol can be used in the conversion of ether with small quantities of conc. H2SO4 as it can be reused in the following processes. Hence, this is called the continuous etherification reaction.
Illustration-
When an equimolar-quantities of ethanol and propanol are heated with conc. H2SO4 then,
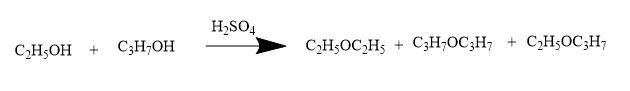
Thus, the products formed are,
C2H5OC2H5, C3H7OC3H7, C2H5OC3H7.
Therefore, option (D) is correct.
Note: When the groups of alcohol are different but are reacting with each other in the presence of conc. H2SO4 then the ether formed will be of three types including groups separately and in combination as well. Ether are ester and would be visible the same when we see the chemical formula. But do remember that they differ as esters have double bonded oxygen within the C atoms and ethers have a single bond. Do not get confused just by looking at the options.
