Question
Question: An engine operates by taking n moles of an ideal gas through the cycle ABCDA shown in the figure. Th...
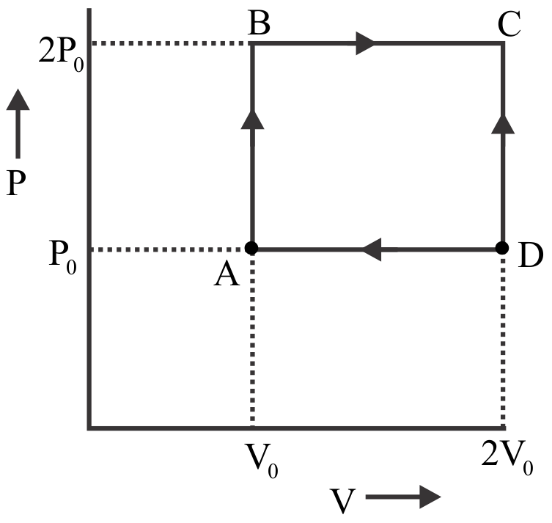
An engine operates by taking n moles of an ideal gas through the cycle ABCDA shown in the figure. The thermal efficiency of the engine is: (TakeCv=1.5R, where R, is gas constant)
(a) 0.32
(b) 0.24
(c) 0.15
(d) 0.08
Solution
Hint Since we have to find thermal efficiency. So for this, first of all, we know the work done will be W=P0V0and also from the ABCDA we can say that the heat given will be equal to the sum of heat produced by Q through ABand heat produced by Q throughBC. And by using the heat formula we can solve this problem.
Formula used:
Work is done,
W=P0V0
Here,
P0, will be the pressure
V0, will be the volume
Heat given will be equal to,
⇒ncvdt+ncpdt
Here,
dt, will be the change in the temperature
cpand cv, will be the pressure and the volume respectively.
Complete Step By Step Solution
As we know the formula of work done which is equal to the
W=P0V0
So from the question, we can write the heat given
Heat given will be equal to,
Heat has given =QAB+QBC
Now by using the heat given formula mentioned above, we can write it as
⇒ncvdtAB+ncpdtBC
As we already know the value of Cv=1.5R
Therefore substituting this value in the heat given equation, we get
Heat gave =23(nRTB−nRTA)+25(nRTC−nRTB)
For the monatomic gas, the value will be 3
Now putting the values we had calculated above, we get
⇒23(2P0V0−P0V0)+25(4P0V0−2P0V0)
On simplifying the solution, we get
⇒213P0V0
As we know the formula for efficiency can be given by
η=heat gainW
It can also be written in the following way and also substituting the values, we get
⇒P0V0213P0V0
On solving,
⇒132
⇒η=0.15
Therefore, the option C will be the correct one.
Note Efficiency can be calculated as a function of many parameters, one of which being temperature or the amount of heat supplied.
Thermal efficiency, therefore, is a measure of how well the machine or device has utilized the energy given to it. The term thermal is used simply for the fact that it is related to the temperature parameter. Efficiencies are also calculated in terms of mechanical advantage obtained called the mechanical efficiency
