Question
Question: An Element X combines with oxygen to form an oxide \({X_2}O\). This oxide is electrically conducting...
An Element X combines with oxygen to form an oxide X2O. This oxide is electrically conducting. Write the formula of the compound which is formed when X reacts with chlorine.
A. XCl2
B. XCl
C. X3Cl2
D. none of the above.
Solution
Both element X and O are combining to form its oxide, here the combination reaction is taking place, the compound formed depends upon the valency of both the elements combining.
Atomic number of oxygen is 8.
Atomic number of chlorine is 17.
We can calculate the valency of X using the given statement and then substitute it to find the required formula of reacting with chlorine.
Complete step by step answer:
Valency of chlorine and oxygen can be given as:
Oxygen:
Atomic number = 8
Electronic arrangement = 2, 6
It can easily take 2 electrons to become stable (8 electrons in valence shell) and hence has:
Valency = 2 ________ (1)
Chlorine:
Atomic number = 17
arrangement = 2, 8, 7
It can easily take 1 electron to become stable (8 electrons in valence shell) and hence has:
Valency = 1 ___________ (2)
Now, the formula of compound formed when two elements combine is given as:
Let A element has valency x and B has valency y.
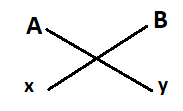
Formula of compound formed = AyBx.
Using this, we can calculate the valency of element X using the given statement:
Element X combines with oxygen to form an oxide X2O.
Therefore, Valency of X = a
Valency of oxygen = 2………………[from (1)]
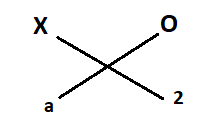
Formula of compound formed should be = X2Oa but
Formula of compound formed is X2O
Comparing the two, we can say that the value of a is 1.
Therefore, the valency of element X is 1.
Now, it combining with chlorine:
Valency of X = 1
Valency of chlorine = 1…………………[from (2)]
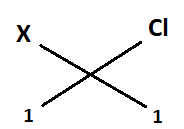
Formula of compound = XCl
Therefore, the formula of the compound which formed when X reacts with chlorine is XCl.
Hence the correct answer is (B).
Note: While solving these kinds of problems, we must look onto: How many electrons would be there in the outermost shell of the element and its valency
The electrons given to first are 2 and then 8 each, so when the outermost shell has 8 valence electrons, the atom is considered to be in the most stable state.
