Question
Question: An element with variable (more than one) stored underwater is: (A) Silicon (B) Iron (C) Phosph...
An element with variable (more than one) stored underwater is:
(A) Silicon
(B) Iron
(C) Phosphorous
(D) Gold
Solution
Element belongs to group 15. It has atomic number 15. It either accepts three electrons or donates five electrons, so it has variable valency. It is found in many allotropic forms white, black and red.
Complete step by step solution:
-Atomic number of phosphorus is 15. The electronic configuration of phosphorus 1s22s22p63s23p3.
-Phosphorus belongs to group 15 of the periodic table. It is a p block.
-Depending on the neighbouring element, it either accepts three electrons or donates five electrons, so it has variable valency.
-Phosphorous exhibits many allotropic forms. White, red and black are important ones.
-White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water. It is soluble in carbon disulphide. It exhibits chemiluminescence. It dissolves in sodium hydroxide in an inert atmosphere giving PH3.
-White phosphorus has less stability and it is more reactive than other solid phases under normal conditions.
-In P4 molecules, angles are 60 degrees, so it leads to angular strain.
-in air, it catches fire readily to give dense white fumes of P4O10.
-to prevent oxidation by oxygen present in the air, phosphorus is stored underwater as it is insoluble water.
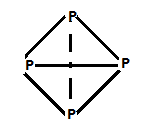
An element with variable (more than one) stored underwater is (C) Phosphorous.
Note: Red phosphorus is obtained at 573k by heating white phosphorus. Common oxidation states of phosphorus are -3 and +5. Valency of phosphorus is 3 and 5. In an excited state, an electron from 3s is transferred to 3d. Phosphorus has the ability to form P-P single bonds.
