Question
Question: An element crystallizes in bcc structure. The edge length of its unit cell is 288 pm. If the density...
An element crystallizes in bcc structure. The edge length of its unit cell is 288 pm. If the density of the crystal is 7.2 g cm−3. what is the atomic mass (in g/mol) of the element?
A. 51.8
B. 103.6
C. 25.9
D. 207.2
Solution
Body-centered cubic unit cell is one kind of cubic lattice. In this cubic lattice each unit cell contains one atom at its body center. And contains one atom at the eight corners of the unit cell.
Formula used: Density = (N×a3)(M×z)= (edge length)3 (Avogadro number)(molecular weight) (number of atoms per cell)
Complete step by step answer:
The structure is shown below,
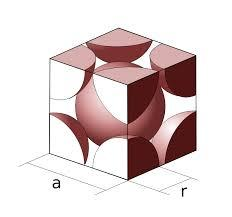
We know that,
Density = (mass)/(volume)
Applying this concept for calculating the density of the unite cell, we get,
Density = (N×a3)(M×z)
= (edge length)3 (Avogadro number)(molecular weight) (number of atoms per cell)
= 7.2=(288×1010)3×6.022×1023M×2
Hence,
Therefore, the molecular weight of the element is 51.77gm/mol.
Additional information:
In the B.C.C the closest distance between two atoms in the unit lattice is23a. Where a is the edge length of the unit lattice.
Now according to this formula 23a = 173pm
Therefore, the edge length is,
Note: The contribution of each corner atoms is 81th of a total atom. So, the contribution of all eight atoms is, 81×8=1.
For the body center atoms, the contribution of that atom is a total of a total atom.
Total number of atoms is 2 in a B.C.C lattice.
