Question
Question: An electron in a hydrogen atom undergoes a transition from an orbit with quantum number \({n_i}\) to...
An electron in a hydrogen atom undergoes a transition from an orbit with quantum number ni to another with quantum number nf . Vi and Vf are respectively the initial and the final potential energies of the electron. If VfVi=6.25 , then the smallest possible nfis
Solution
Use the relation between the quantum number of the electron and its potential energy and substitute the given ratio of the potential energy in it. In order to find the smallest possible final quantum number, the largest possible initial quantum number is taken.
Useful formula:
The relation between the quantum number and the potential energy is given by
VfVi=ni2nf2
Where Vi is the initial potential energy of the electron, ni is the initial quantum number of the electron and nf is the final quantum number.
Complete step by step solution:
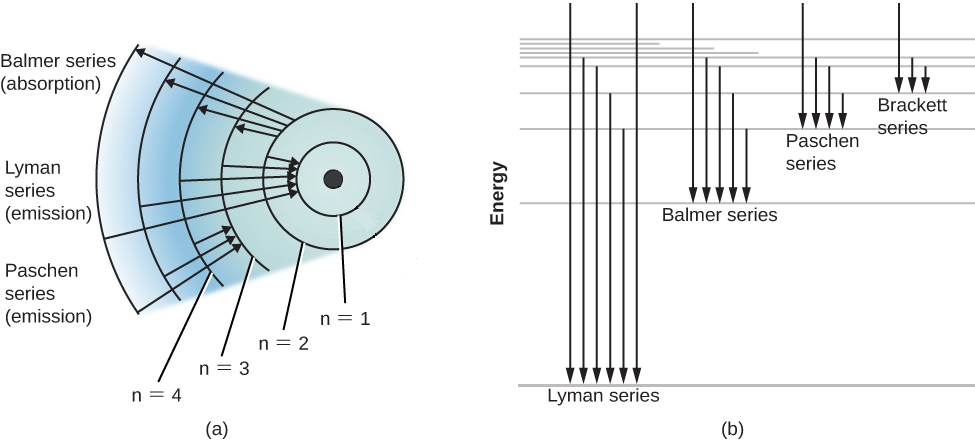
It is given that the
Ratio of the initial and the final potential energy is, VfVi=6.25
Using the relation given above,
VfVi=ni2nf2
Substituting the values of the VfVi in the above relation,
ni2nf2=6.25
Taking square root on both sides of the equation
ni2nf2=6.25
ninf=2.5
The initial quantum number cannot be 1 , so it is taken as 2 .
Substituting the values of the ni in the above equation.
nf=2.5×ni
nf=2.5×2
By performing the simple arithmetic operations,
nf=5
Thus, the value of the final quantum number is obtained as 5.
Note: Note that when the electron of the hydrogen atoms migrates from the small quantum levels to the large quantum levels, it absorbs some energy. And this process is called ionizing. But when the electron moves to the lower from the higher quantum levels, it releases some energy.
