Question
Question: An atom that has electronic configuration \(1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^3}4{s^2}\) , you will ...
An atom that has electronic configuration 1s22s22p63s23p63d34s2 , you will place in which of the following group:
A. Fifth
B. Fifteen
C. Second
D. Third
Solution
Electronic configuration is defined as the way in which the electrons are distributed in its atomic orbitals. It follows a standard notation where all the electrons containing subshells are placed in a sequence.
Complete step by step answer:
Electronic configuration is defined as the way in which the electrons are distributed in its atomic orbitals.
There is a standard notation which it follows where all the electrons containing subshells are placed in a sequence.
The standard notation of electronic configuration is given below:
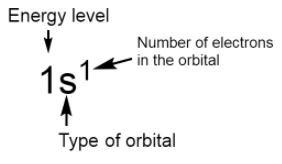
General electronic configurations of different blocks are given below:
1.Alkali metals: ns1
2.Alkali earth metals: ns2
3.P block elements: ns2np1−6
4.D-block elements: (n−1)d1−10ns1−2
5.F block elements: (n−2)f0−14(n−1)d0−1ns2
To check the group of the atom which it belongs to through the electronic configuration then we have to add the electrons from the outermost shell and the shell prior to it.
The outermost shell and the one shell prior to it that is d orbital consists of total 5 electrons which means that the atom belongs to the d block.
d- block starts from group 3 to group 12 .
So, option b) fifteen and option c) second will be eradicated because group 15 comes under the p block and group 2 comes under the s block.
Now, as we know there are a total of 5 electrons in their outermost shell and the shell prior to it, so the above atom belongs to the group 5 of d block elements.
So, the correct answer is Option A.
Note: We will never get to know the exact group of an element just by the electrons that are present in their outermost shell. The electrons in the outermost shell will only let you know in which block it
