Question
Question: An aromatic compound A of the molecular formula \({{C}_{8}}{{H}_{10}}O\) on reaction with iodine and...
An aromatic compound A of the molecular formula C8H10O on reaction with iodine and dilute NaOH gives a yellow precipitate. The structure of the compound is expected to be:
A.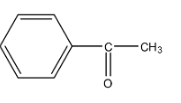
B. C6H5−CHOH−CH3
C.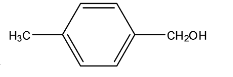
D.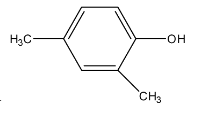
Solution
The compound has molecular formula C8H10O, since it has one oxygen, then the compound will be alcohol, an ester, or a ketone. The second information given is that the compound forms a yellow precipitate with iodine and NaOH, this means that the compound gives the Iodoform test.
Complete step by step solution: The compound has molecular formula C8H10O, since it has one oxygen, then the compound will be alcohol, an ester, or a ketone. The second information given is that the compound forms a yellow precipitate with iodine and NaOH, this means that the compound gives the Iodoform test.
So, the compounds that will positive test for the Iodoform test when it give a yellow precipitate with iodine and dilute NaOH, and there is a condition for the compound to give the Iodoform test is the presence of −COCH3 group.
So, in the given options there are two compounds in which the −COCH3 group is present, i.e., option (a) and option (b). The molecular formula of the compound in option (a) is C8H8O and the molecular formula of the compound in option (b) is C8H10O.
So, the reaction is given below:
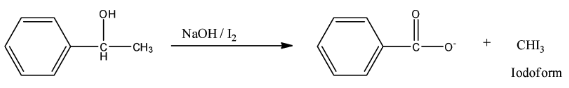
So, this compound is secondary alcohol and gives yellow precipitate in the Iodoform test.
Therefore, the correct answer is an option (b).
Note: Iodoform is an important test that is used to distinguish between methyl ketone and non-methyl ketones. A compound that doesn't give the Iodoform test is formaldehyde or methanal.
