Question
Question: An aromatic compound A of molecular formula \({{\text{C}}_{\text{7}}}{{\text{H}}_{\text{7}}}{\text{O...
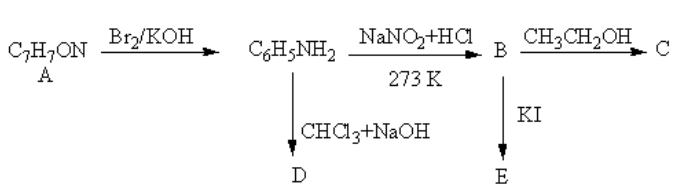
An aromatic compound A of molecular formula C7H7ON undergoes a series of reaction as shown below. Write the structures of A, B, C, D and E in the following reactions.
Solution
The reagent Br2/KOH is used to convert the amide functional group into amine. The reagent NaNO2 + HCl is used to convert the amine functional group into diazonium ion. The reagent CH3CH2OH adds the proton to cation formed by diazonium ion. The reagent CHCl3 + NaOH is used to convert the amine functional group into cyanide. The reagent KI is used to add iodine.
Complete answer:
The reagent Br2/KOH converts the amide functional group into amine so, the compound A should be an amide. The structure of A and its reaction is as follows:
The amide reacts with bromine and potassium hydroxide to give amine. The reaction is known as the Hoffmann bromamide reaction.
Initially, the base sodium hydroxide abstract proton form acetamide to generate an anion of amide
The anion of amide now attacks on bromine, so an N-bromoamide generates.
Again the base attacks on N-bromoamide so an anion of bromoamide forms.
Then the methyl group attached with the carbonyl group shifts to the nitrogen atom and bromide ion leaves forming an isocyanate structure.
Nucleophilic attack of water on isocyanate takes place which loses the carbon dioxide and forms a structure in which nitrogen has a negative charge and one hydrogen and one benzene group.
Then the protonation of this nitrogen takes place which gives aniline.
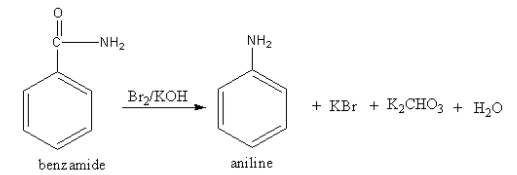
So, A is benzamide.
The reagent NaNO2 + HClconverts the amine functional group into diazonium ion. The product of reaction of aniline withNaNO2 + HCl is as follows:
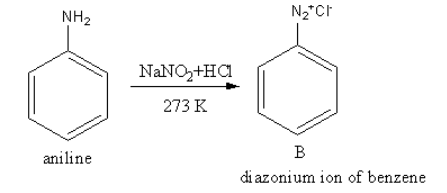
So, B is a diazonium salt of benzene.
The reagent CH3CH2OH is used for protonation of benzene cation. The product of reaction of diazonium salt of benzene with CH3CH2OH is as follows:
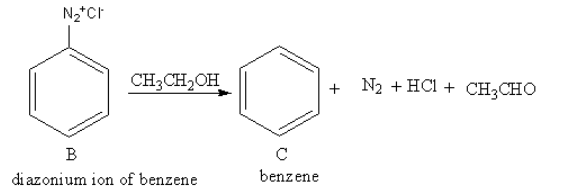
So, C is benzene.
The reagent CHCl3 + NaOHconverts the amine functional group into cyanide. The conversion of the amine functional group into the isocyanide functional group by the reaction of primary amine with chloroform and base is known as Carbylamine reaction.
The product of reaction of aniline withCHCl3 + NaOH is as follows:
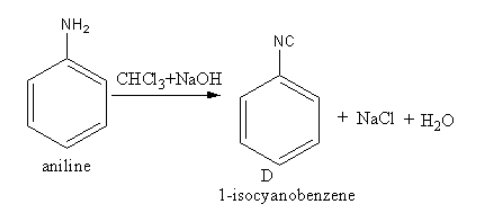
So, D is phenyl isocyanide also known as 1−isocyanatobenzene.
The reagent KI is used to add iodine. The reaction of benzene with potassium iodide is a nucleophilic substitution reaction of benzene.
The product of the reaction of benzene with potassium iodide is as follows:
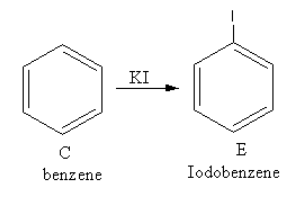
So, E is iodobenzene.
**Therefore, A , B ,C, D and E are benzamide, diazonium salt, benzene, phenyl isocyanide and iodobenzene respectively.
Note:**
The Reagent used in the Carbylamine reaction is chloroform and a base. The Carbylamine reaction is also known as Hofmann isocyanide synthesis. The reagent used in the Hoffmann bromamide reaction is base.
