Question
Question: An aqueous solution of X is added slowly to an aqueous solution of Y as shown in List I. The variati...
An aqueous solution of X is added slowly to an aqueous solution of Y as shown in List I. The variation in conductivity of these reactions is given in List II. Match List I with List II and select the correct answer using the code given below the lists :
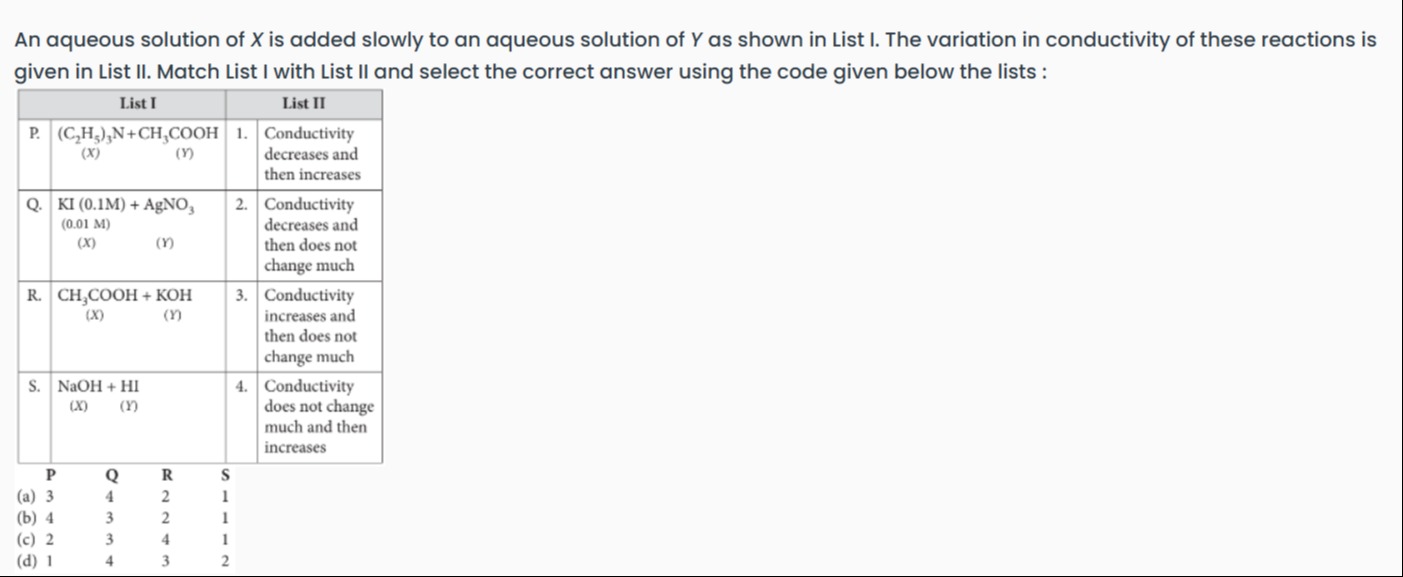
3 4 2 1
4 3 2 1
2 3 4 1
1 4 3 2
Option (a): P → 3, Q → 4, R → 2, S → 1.
Solution
We can “picture‐titrate” the conductivity changes by noticing that the order in which the ions of high and low mobility appear (or disappear) differs from reaction to reaction.
Let X be the titrant and Y be in the flask. In our four cases:
• For reaction P
X = (C₂H₅)₃N (a weak base) and Y = CH₃COOH (a weak acid).
An aqueous solution of a weak acid has a very low conductivity (because it ionizes only slightly). When (C₂H₅)₃N is added, the acid is neutralized to form the salt (triethylammonium acetate) which, though itself a weak electrolyte, dissociates slightly “better” than CH₃COOH so the net conductivity increases (but only modestly) and then “levels‐off” once the salt is completely formed.
Thus the curve “rises (increases) and then remains nearly constant”. This is described by List II option 3.
• For reaction Q
X = KI and Y = AgNO₃.
AgNO₃ is a strong electrolyte so initially the solution is reasonably conductive. On adding KI, Ag⁺ is removed as insoluble AgI; however, the replacement (by K⁺, while NO₃⁻ remains) means that initially the net conductivity does not change much. Beyond the “end‐point” the extra KI (a good conductor) makes the conductivity rise sharply.
This two‐phase behavior is given by option 4.
• For reaction R
X = CH₃COOH and Y = KOH.
Here the flask solution is KOH (a strong electrolyte giving high conductivity mainly because of OH⁻, which is highly mobile). As CH₃COOH is added, OH⁻ is progressively neutralized to give CH₃COO⁻ (which has low mobility) so the conductivity falls. Once neutralization is complete further addition of CH₃COOH (a weak electrolyte) produces little extra conductivity.
This gives “conductivity decreases and then does not change much” which is option 2.
• For reaction S
X = NaOH and Y = HI.
HI is a strong acid; its solution has a very high conductivity mainly due to H⁺ (extremely high ionic mobility). When NaOH is added, H⁺ is “removed” (and replaced by Na⁺ which has a much lower mobility) causing a steep fall in conductivity. Beyond the equivalence point the excess NaOH increases the conductivity.
Thus the conductivity “decreases initially, then increases” which is described by option 1.
So the final matching is:
P → 3, Q → 4, R → 2, S → 1.
Looking at the answer codes given the four rows, this correspondence is represented by option (a): 3 4 2 1.
Quick (Bare‐bone) Explanation
- In titration P (weak–weak) the salt formed is slightly more ionized than CH₃COOH so after a small increase the conductivity levels off → option 3.
- In Q (precipitation) removal of Ag⁺ makes little change until excess KI is added → option 4.
- In R (weak acid–strong base) OH⁻ (highly conducting) is replaced by CH₃COO⁻ (poorly conducting) so the conductivity drops and then barely changes → option 2.
- In S (strong acid–strong base) high‐mobility H⁺ is replaced by Na⁺ causing an initial drop and later excess OH⁻ raises the conductivity → option 1.
