Question
Question: An amount of 19 g of molten $SnCl_2$ is electrolysed for some time. Inert electrodes are used. 1.19 ...
An amount of 19 g of molten SnCl2 is electrolysed for some time. Inert electrodes are used. 1.19 g of tin is deposited at the cathode. No substance is lost during electrolysis. If the ratio of the masses of SnCl2 and SnCl4 after electrolysis is x:261, the value of x is (Sn = 119)
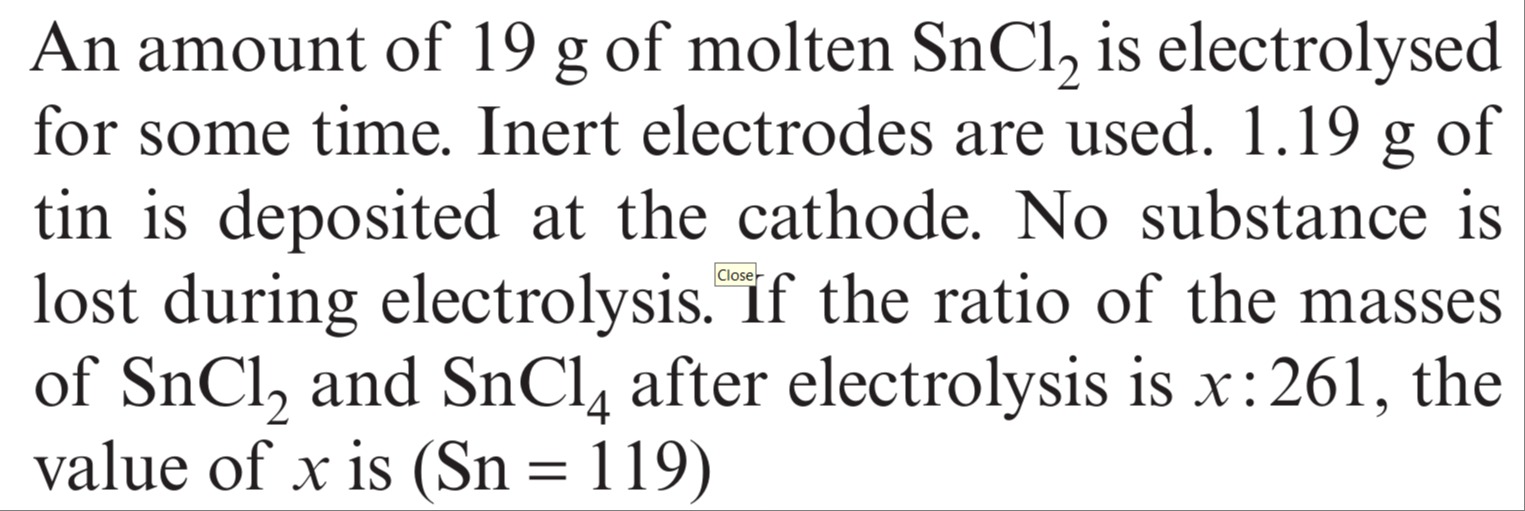
1520
15.20
261
119
1520
Solution
The electrolysis of molten SnCl2 results in the formation of tin metal at the cathode and SnCl4 at the anode, via the disproportionation reaction: 2SnCl2→SnCl4+Sn
From the stoichiometry: 2 moles of SnCl2 (2 × 190 g = 380 g) yield 1 mole of SnCl4 (261 g) and 1 mole of Sn (119 g).
Given that 1.19 g of tin is deposited: Moles of Sn deposited = 119 g/mol1.19 g=0.01 mol.
Based on the stoichiometry:
-
Mass of SnCl4 produced: Since 1 mole of Sn is produced with 1 mole of SnCl4, 0.01 mol of SnCl4 is produced. Mass of SnCl4 = 0.01 mol×261 g/mol=2.61 g.
-
Mass of SnCl2 reacted: Since 2 moles of SnCl2 react to produce 1 mole of Sn, 0.02 mol of SnCl2 reacted. Mass of SnCl2 reacted = 0.02 mol×190 g/mol=3.80 g.
-
Mass of SnCl2 remaining: Initial mass of SnCl2 = 19 g. Mass of SnCl2 remaining = 19 g−3.80 g=15.20 g.
-
Determine the ratio: The ratio of the masses of SnCl2 and SnCl4 after electrolysis is given as x:261. This ratio compares the remaining SnCl2 to the produced SnCl4. The calculated ratio is 15.20 g:2.61 g.
-
Solve for x: We set up the proportion: 2.6115.20=261x x=2.6115.20×261=15.20×100=1520.
