Question
Question: An amount of 1 mole of PCl3(g) and 1mole of PCl5(g) is taken in a vessel of 10L capacity maintained ...
An amount of 1 mole of PCl3(g) and 1mole of PCl5(g) is taken in a vessel of 10L capacity maintained at 400 K. At equilibrium, the moles of Cl2 is found to be 0.004.
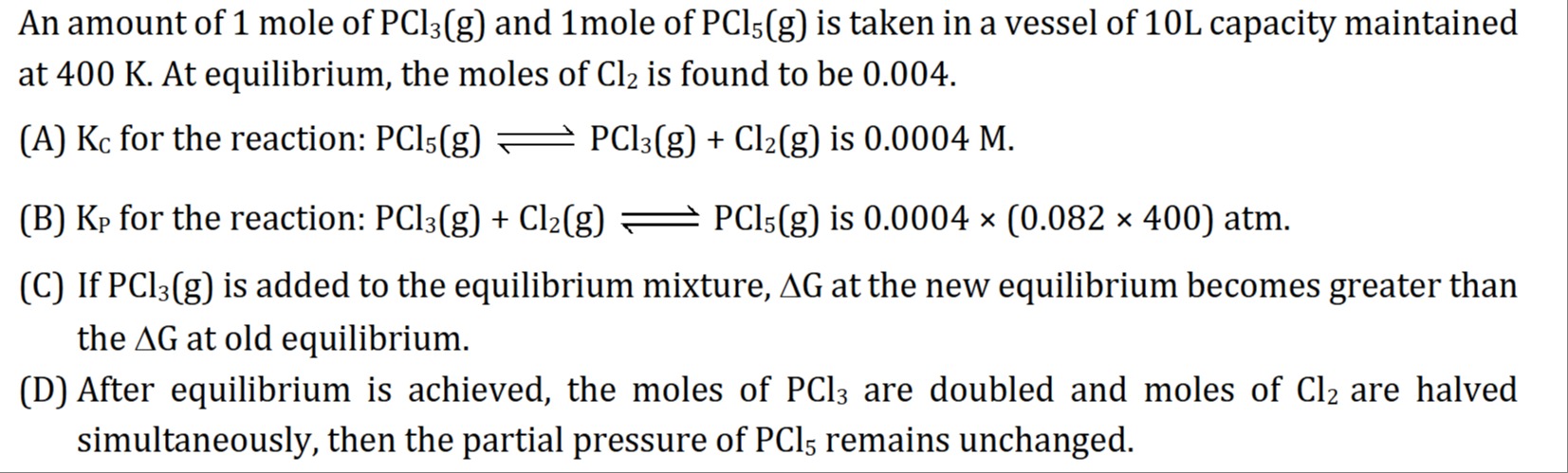
Kc for the reaction: PCl5(g) ⇌ PCl3(g) + Cl2(g) is 0.0004 M.
Kp for the reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) is 0.0004 × (0.082 × 400) atm.
If PCl3(g) is added to the equilibrium mixture, ΔG at the new equilibrium becomes greater than the ΔG at old equilibrium.
After equilibrium is achieved, the moles of PCl3 are doubled and moles of Cl2 are halved simultaneously, then the partial pressure of PCl5 remains unchanged.
D
Solution
The reaction is: PCl5(g) ⇌ PCl3(g) + Cl2(g)
Given initial conditions: Moles of PCl3 = 1 mole Moles of PCl5 = 1 mole Moles of Cl2 = 0 mole (initially) Volume of vessel (V) = 10 L Temperature (T) = 400 K
At equilibrium, moles of Cl2 = 0.004. Let 'x' be the change in moles of PCl5.
| Species | Initial Moles | Change | Equilibrium Moles | Equilibrium Concentration (mol/L) |
|---|---|---|---|---|
| PCl5 | 1 | -x | 1 - x | (1 - x)/10 |
| PCl3 | 1 | +x | 1 + x | (1 + x)/10 |
| Cl2 | 0 | +x | x | x/10 |
From the problem, at equilibrium, moles of Cl2 = 0.004. So, x = 0.004.
Equilibrium moles: PCl5 = 1 - 0.004 = 0.996 moles PCl3 = 1 + 0.004 = 1.004 moles Cl2 = 0.004 moles
Equilibrium concentrations: [PCl5] = 0.996 mol / 10 L = 0.0996 M [PCl3] = 1.004 mol / 10 L = 0.1004 M [Cl2] = 0.004 mol / 10 L = 0.0004 M
Evaluate Statement (A): Kc for the reaction: PCl5(g) ⇌ PCl3(g) + Cl2(g) is 0.0004 M.
The expression for Kc is: Kc=[PCl5][PCl3][Cl2] Substitute the equilibrium concentrations: Kc=(0.0996)(0.1004)(0.0004) Kc=0.09960.00004016 Kc≈0.0004032M
The statement says Kc is 0.0004 M. Our calculated value is 0.0004032 M. This is a very close approximation, likely rounded.
Evaluate Statement (B): Kp for the reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) is 0.0004 × (0.082 × 400) atm.
First, let's find Kp for the forward reaction: PCl5(g) ⇌ PCl3(g) + Cl2(g). The relationship between Kp and Kc is: Kp=Kc(RT)Δng Here, Δng = (moles of gaseous products) - (moles of gaseous reactants) = (1 + 1) - 1 = 1. So, Kp(forward)=KcRT Using Kc≈0.0004032 M, R = 0.082 L atm mol⁻¹ K⁻¹, T = 400 K: Kp(forward)=0.0004032×0.082×400=0.0004032×32.8≈0.01323 atm.
The statement asks for Kp for the reverse reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g). Let this be Kp′. Kp′=Kp(forward)1 Kp′=0.013231≈75.58 atm⁻¹. The statement says Kp′ is 0.0004×(0.082×400)=0.01312 atm. This value is approximately Kp for the forward reaction, not the reverse reaction. Also, the unit is incorrect for the reverse reaction (it should be atm⁻¹). Therefore, statement (B) is incorrect.
Evaluate Statement (C): If PCl3(g) is added to the equilibrium mixture, ΔG at the new equilibrium becomes greater than the ΔG at old equilibrium.
At any equilibrium, by definition, the Gibbs free energy change (ΔG) for the system is zero. When PCl3 is added to an equilibrium mixture, the system is no longer at equilibrium. It will shift to a new equilibrium state according to Le Chatelier's principle (in this case, to the left, favoring PCl5 formation). However, once the new equilibrium is established, ΔG will again be zero. Therefore, ΔG at the new equilibrium is equal to ΔG at the old equilibrium (both are 0). Statement (C) is incorrect.
Evaluate Statement (D): After equilibrium is achieved, the moles of PCl3 are doubled and moles of Cl2 are halved simultaneously, then the partial pressure of PCl5 remains unchanged.
Let the initial equilibrium moles be nPCl5,eq, nPCl3,eq, nCl2,eq. We know that Kc=[PCl5]eq[PCl3]eq[Cl2]eq=(nPCl5,eq/V)(nPCl3,eq/V)(nCl2,eq/V)=nPCl5,eqVnPCl3,eqnCl2,eq.
Now, consider the changes: New moles of PCl3 (nPCl3′) = 2×nPCl3,eq New moles of Cl2 (nCl2′) = 0.5×nCl2,eq Moles of PCl5 (nPCl5′) = nPCl5,eq (as it is not explicitly changed by the operation)
Let's calculate the reaction quotient (Qc) immediately after these changes, before any reaction occurs: Qc=[PCl5]′[PCl3]′[Cl2]′=(nPCl5′/V)(nPCl3′/V)(nCl2′/V) Qc=(nPCl5,eq/V)(2nPCl3,eq/V)(0.5nCl2,eq/V) Qc=nPCl5,eqV(2×0.5)nPCl3,eqnCl2,eq=nPCl5,eqVnPCl3,eqnCl2,eq
Since Qc=Kc, the system is already at equilibrium after these changes. No net reaction will occur. This means the moles of PCl5 remain nPCl5,eq.
For a gas in a fixed volume and temperature, the partial pressure is directly proportional to its moles: Pi=VniRT Since the moles of PCl5 (nPCl5), R, T, and V are unchanged from the initial equilibrium state, the partial pressure of PCl5 will also remain unchanged. Therefore, statement (D) is correct.
Comparing (A) and (D), (D) is precisely correct based on the principle that Q=K implies no change, and partial pressure is proportional to moles. (A) is an approximation. In competitive exams, if one option is precisely correct and another is an approximation, the precise one is the intended answer.
The final answer is D
Explanation of the solution:
-
Calculate Equilibrium Concentrations: Use the initial moles and the given equilibrium moles of Cl2 to determine the equilibrium moles of all species using an ICE table. Then, divide by the volume (10 L) to get concentrations.
- Initial: PCl5=1 mol, PCl3=1 mol, Cl2=0 mol.
- Change: PCl5=-x, PCl3=+x, Cl2=+x.
- Equilibrium: PCl5=1-x, PCl3=1+x, Cl2=x.
- Given x = 0.004 mol.
- Equilibrium moles: PCl5=0.996 mol, PCl3=1.004 mol, Cl2=0.004 mol.
- Equilibrium concentrations: [PCl5]=0.0996 M, [PCl3]=0.1004 M, [Cl2]=0.0004 M.
-
Evaluate Statement (A): Calculate Kc using the equilibrium concentrations.
- Kc=[PCl5][PCl3][Cl2]=(0.0996)(0.1004)(0.0004)≈0.0004032 M.
- Statement (A) says Kc = 0.0004 M, which is an approximation.
-
Evaluate Statement (B): Calculate Kp for the forward reaction using Kp=Kc(RT)Δng, where Δng=1. Then, find Kp for the reverse reaction as 1/Kp(forward).
- Kp(forward)=0.0004032×(0.082×400)≈0.01323 atm.
- Kp(reverse)=1/Kp(forward)≈75.58 atm⁻¹.
- Statement (B) gives a value of 0.0004×(0.082×400)=0.01312 atm, which is approximately Kp for the forward reaction, not the reverse, and the unit is incorrect. So, (B) is false.
-
Evaluate Statement (C): Recall that at any equilibrium, ΔG=0. Adding a component shifts the equilibrium, but at the new equilibrium, ΔG will again be zero. So, (C) is false.
-
Evaluate Statement (D): Consider the effect of doubling moles of PCl3 and halving moles of Cl2 on the reaction quotient Q.
- Initial equilibrium moles: nPCl3,nCl2,nPCl5.
- New state moles: nPCl3′=2nPCl3, nCl2′=0.5nCl2, nPCl5′=nPCl5 (unchanged by the stated operation).
- Calculate Qc=(nPCl5′/V)(nPCl3′/V)(nCl2′/V)=(nPCl5/V)(2nPCl3/V)(0.5nCl2/V)=nPCl5VnPCl3nCl2.
- This expression for Qc is identical to Kc. Therefore, the system is still at equilibrium.
- Since the system is at equilibrium and the moles of PCl5 were not changed by the operation, and partial pressure is directly proportional to moles (Pi=niRT/V), the partial pressure of PCl5 remains unchanged. So, (D) is correct.
