Question
Question: An alkyl halide with $\beta$-hydrogen atoms, when treated with a nucleophilic base, undergo a substi...
An alkyl halide with β-hydrogen atoms, when treated with a nucleophilic base, undergo a substitution reaction or an elimination reaction, depending on the strength and size of the nucleophile base and the reaction conditions.
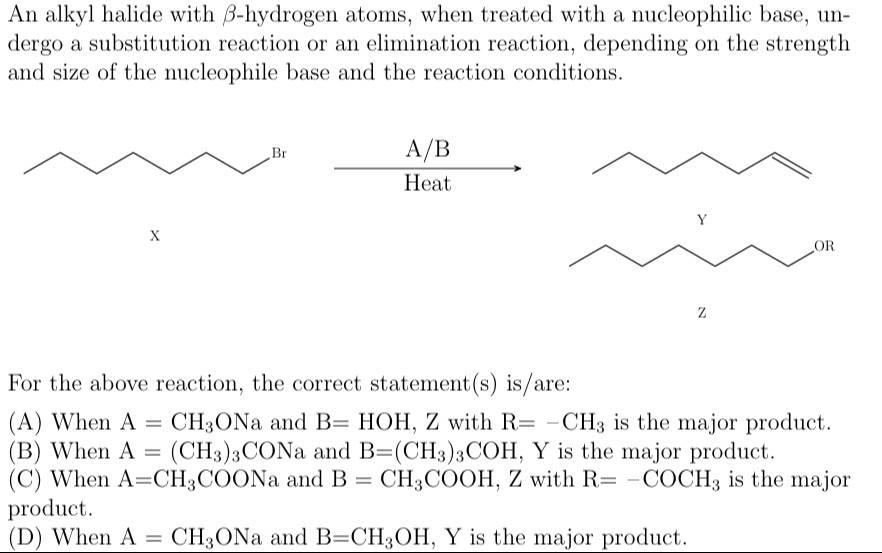
When A = CH3ONa and B= HOH, Z with R= -CH3 is the major product.
When A = (CH3)3CONa and B=(CH3)3COH, Y is the major product.
When A=CH3COONa and B = CH3COOH, Z with R= -COCH3 is the major product.
When A = CH3ONa and B=CH3OH, Y is the major product.
B,C
Solution
The given reaction involves a primary alkyl halide, 1-bromohexane (X), reacting with various nucleophilic bases (A) in different solvents (B) under heating conditions. The possible products are an alkene (Y, 1-hexene) via elimination (E2) and an ether/ester (Z, 1-alkoxyhexane or 1-acyloxyhexane) via substitution (SN2).
Let's analyze each option:
General considerations for primary alkyl halides:
- Primary alkyl halides are highly susceptible to SN2 reactions due to low steric hindrance at the reaction center.
- E2 reactions are possible, especially with strong bases and elevated temperatures.
- SN1 and E1 reactions are generally not favored for primary alkyl halides because primary carbocations are highly unstable.
Analysis of options:
(A) When A = CH3ONa and B= HOH, Z with R= -CH3 is the major product.
- Reagents: Sodium methoxide (CH3ONa) is a strong base and a strong nucleophile. However, it reacts with water (HOH), which is a protic solvent: CH3ONa + H2O → CH3OH + NaOH Therefore, the effective nucleophile/base in this system is hydroxide ion (OH-) in water.
- Reaction: 1-bromohexane (primary alkyl halide) reacts with OH- (strong nucleophile/strong base) in H2O (protic solvent) with heat.
- SN2 reaction would yield 1-hexanol (Z with R = -H, i.e., -OH).
- E2 reaction would yield 1-hexene (Y).
- Conclusion: The statement claims Z with R = -CH3 (1-methoxyhexane) is the major product. This product would form if CH3O- acted as the nucleophile. Since CH3O- is largely converted to OH- in water, 1-methoxyhexane cannot be the major product. Thus, statement (A) is incorrect.
(B) When A = (CH3)3CONa and B=(CH3)3COH, Y is the major product.
- Reagents: Sodium tert-butoxide ((CH3)3CONa) is a strong, bulky base and a poor nucleophile (due to steric hindrance). tert-Butanol ((CH3)3COH) is a polar protic solvent commonly used with bulky bases to favor elimination.
- Reaction: 1-bromohexane (primary alkyl halide) reacts with a strong, bulky base with heat.
- Outcome: Bulky bases preferentially abstract a β-hydrogen, leading to E2 elimination, even for primary alkyl halides, because SN2 attack is sterically hindered. The presence of heat further favors elimination.
- Conclusion: Therefore, 1-hexene (Y) via E2 elimination is expected to be the major product. Thus, statement (B) is correct.
(C) When A=CH3COONa and B = CH3COOH, Z with R= -COCH3 is the major product.
- Reagents: Sodium acetate (CH3COONa) provides the acetate ion (CH3COO-), which is a weak nucleophile and a weak base. Acetic acid (CH3COOH) is a polar protic solvent.
- Reaction: 1-bromohexane (primary alkyl halide) reacts with a weak nucleophile/weak base with heat.
- Outcome:
- Since acetate is a weak base, E2 elimination is highly disfavored.
- SN2 reaction is possible for primary alkyl halides with weak nucleophiles, leading to the formation of an ester. The product would be 1-hexyl acetate (Z with R = -COCH3).
- SN1/E1 are not favored for primary alkyl halides.
- Conclusion: Among the possible reactions, SN2 leading to the ester is the most plausible pathway for a significant product. Therefore, Z with R = -COCH3 (1-hexyl acetate) is expected to be the major product. Thus, statement (C) is correct.
(D) When A = CH3ONa and B=CH3OH, Y is the major product.
- Reagents: Sodium methoxide (CH3ONa) is a strong nucleophile and a strong base. Methanol (CH3OH) is a polar protic solvent, which is the conjugate acid of the nucleophile/base. This is a common setup for Williamson ether synthesis (SN2) or E2.
- Reaction: 1-bromohexane (primary alkyl halide) reacts with a strong, non-bulky nucleophile/base (CH3O-) in a protic solvent with heat.
- Outcome: For primary alkyl halides, SN2 reaction (ether formation) is generally favored over E2 elimination (alkene formation) when using strong, unhindered bases like methoxide, even with heat. The SN2 reaction is very efficient for primary halides.
- Conclusion: Therefore, 1-methoxyhexane (Z with R = -CH3) would be the major product via SN2. The statement claims Y (1-hexene) is the major product, which implies E2 is preferred. This is incorrect for a primary halide with a non-bulky strong base. Thus, statement (D) is incorrect.
Based on the analysis, statements (B) and (C) are correct.
