Question
Question: An alkene “A” on reaction with \({{O}_{3}}\) and \(Zn-{{H}_{2}}O\) gives propanone and ethanol in eq...
An alkene “A” on reaction with O3 and Zn−H2O gives propanone and ethanol in equimolar ratio. The addition of HCl to alkene "A" gives "B" as the major product. The structure of the product "B" is:
(A) 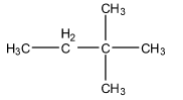
(B) 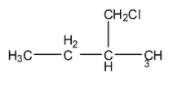
(C) 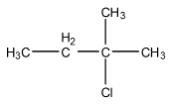
(D) 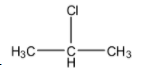
Solution
Alkene is a compound in which there is a double bond and when it is treated with ozone the double bond will break and will form two compounds having the carbonyl group. In a double, the chloride ion can attack either side, but the major product will have more stability.
Complete step by step solution:
The alkene is a compound in which there is a double bond and when it is treated with ozone the double bond will break and will form two compounds having the carbonyl group. And the question is given that the two products formed are propanone and ethanol.
The structure of propanone is given below:
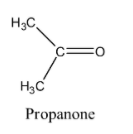
The structure of ethanol is given below:
CH3−CHO
So, the alkene will be 2-Methyl but-2-ene and its structure is given below:
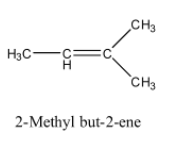
The reaction of 2-Methyl but-2-ene with O3 and Zn−H2O is given below:
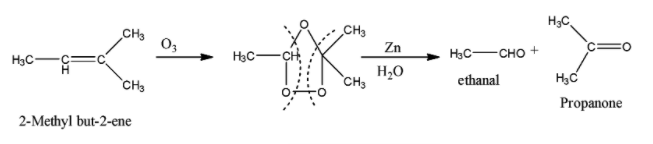
So when 2-Methyl but-2-ene is reacted with HCl, the addition of chloride ion takes place in such a manner that the major product is the one in which the chlorine atom is attached to the tertiary carbon atom. So the reaction is given below:
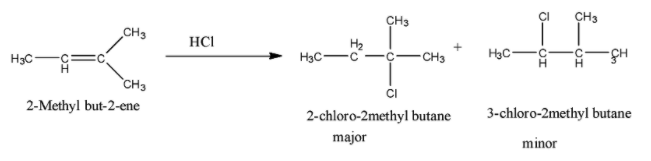
Therefore, the major product is 2-chloro-2methyl methyl butane.
Hence, the correct answer is an option (C).
Note: It must be noted that the addition of HClto the alkene takes place through Markovnikov’s rule i.e., the negative part of the reagent will get attached to the carbon atom having a lesser number of hydrogen atoms.
