Question
Question: An aldohexose can exist in \(16\) isomeric forms. A: True B: False...
An aldohexose can exist in 16 isomeric forms.
A: True
B: False
Solution
Isomers are the chemical compounds that have the same molecular formula but arrangement of atoms is different. Isomers are of many types. Aldohexose is a hexose which has an aldehyde group on one end. Hexose is a monosaccharide with six carbon atoms.
Complete step by step answer:
Isomers are the chemical species which have the same molecular formula but different structures. Aldohexose is a chemical compound in which there is an aldehyde group at one end of hexose. Hexose is a monosaccharide with six carbon atoms. Monosaccharide is the simple sugar. General formula of monosaccharide is (CH2O)n. Aldehyde is a fuctional group with chemical formula −OH. Chemical formula of aldohexose is H−C(=O)−(CHOH)5−H. Glucose is the most common example of aldohexose. Glucose is made by plants during photosynthesis from water and carbon dioxide taking energy from sunlight. Glucose is the subcategory of carbohydrates. Isomers of aldohexose are as follows:
1. D−Allose
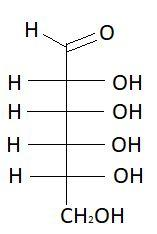
2. L−Allose
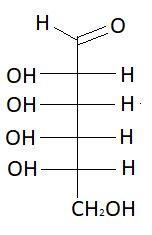
3. D−Altrose
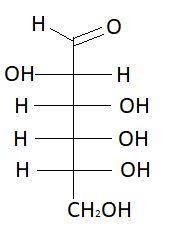
4. L− Altrose
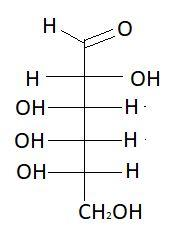
5. D−Glucose
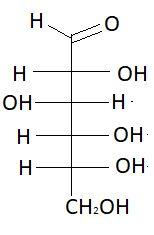
6. L− Glucose
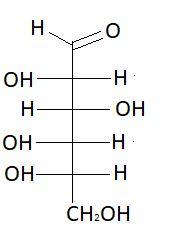
7. D−Mannose
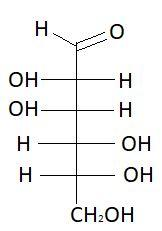
8. L− Mannose
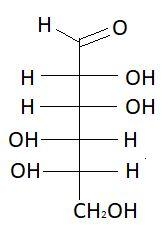
9. D−Gulose
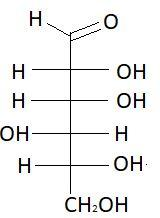
10. L− Gulose
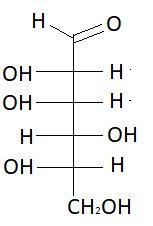
11. D−Idose
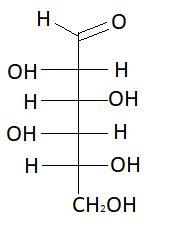
12. L− Idose
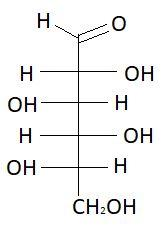
13. D−Galactose
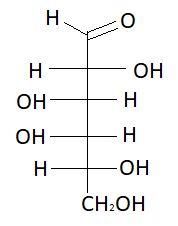
14. L− Galactose
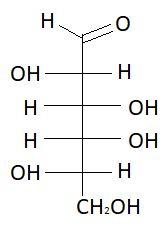
15. D−Talose
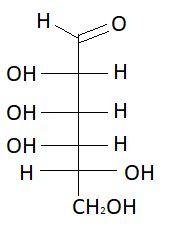
16. L−Talose
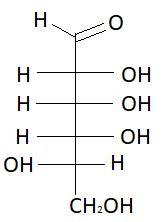
These are the isomers of aldohexose. There are total 16 isomers of aldohexose. The statement states that an aldohexose can exist in 16 isomeric forms, which is true.
Note:
In the name of the isomers of aldohexose, the terms L and D are present. D represents that the compound is dextrorotatory, that is the compound has the ability to rotate planes of polarized light in the right direction. L represents levorotatory meaning that the compound has the ability to rotate planes polarized in the left direction.
