Question
Question: An alcohol X when treated with hot conc. \({H_2}S{O_4}\) gave an alkene Y with formula \({C_4}{H_8}\...
An alcohol X when treated with hot conc. H2SO4 gave an alkene Y with formula C4H8 . This alkene on ozonolysis gives a single product with molecular formula C2H4O . The alcohol is
A.Butan −1− ol
B.Butan −2− ol
C.2− methylpropan −1− ol
D.2,2− dimethylbutan −1− ol
Solution
Ozonolysis reaction is used to identify the position of double bonds in alkenes or other unsaturated compounds. In an ozonolysis reaction, the substrate reacts with an ozone molecule to give ozonide which when further treated with zinc gives the final product. The final product can be a ketone or aldehyde.
Complete step by step answer:
Definition: Ozonolysis is a reaction in which an ozone molecule is added to the unsaturated bond in a molecule to form ozonide and then this ozonide is cleaved or broken into smaller molecules. This reaction is usually carried out with alkenes.
In ozonolysis the carbon-carbon bond in alkenes or alkynes is replaced by a carbonyl group.
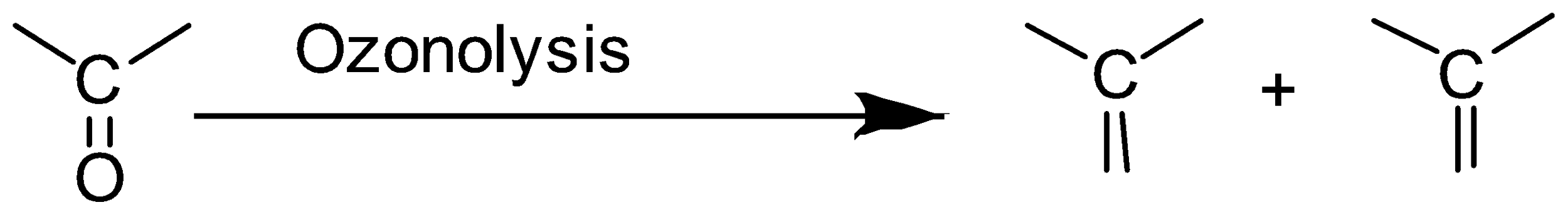
According to the given question,
X(ROH)+H2SO4→Y(C4H8)ozonolysisC2H4O
Now the alkene which is formed in the given reaction is C4H8 . So the possible alkene are
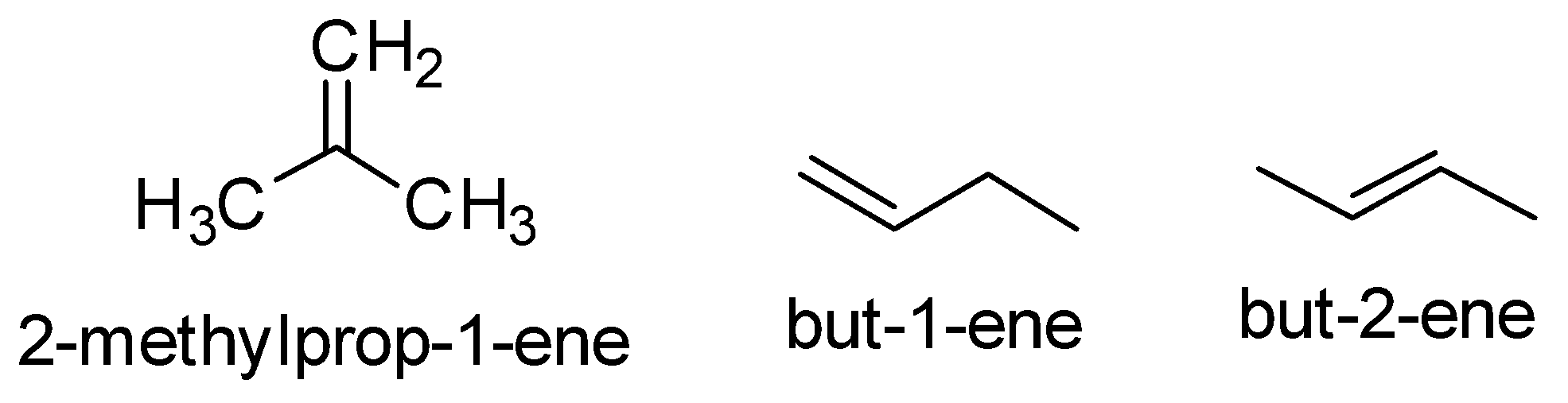
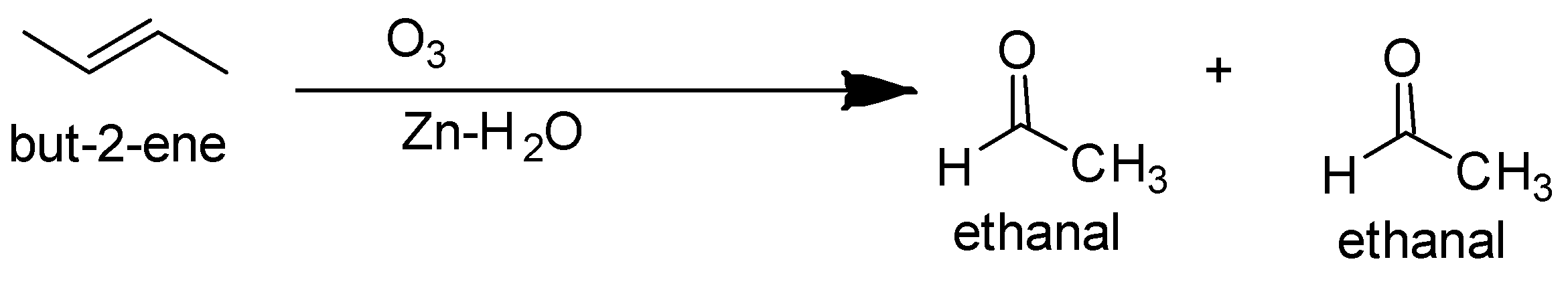
So the ozonolysis of only but −2− ene gives a single product. The reaction is as follows-
The ozonolysis of but −1− ene and 2− methylprop −1− ene are as follows-
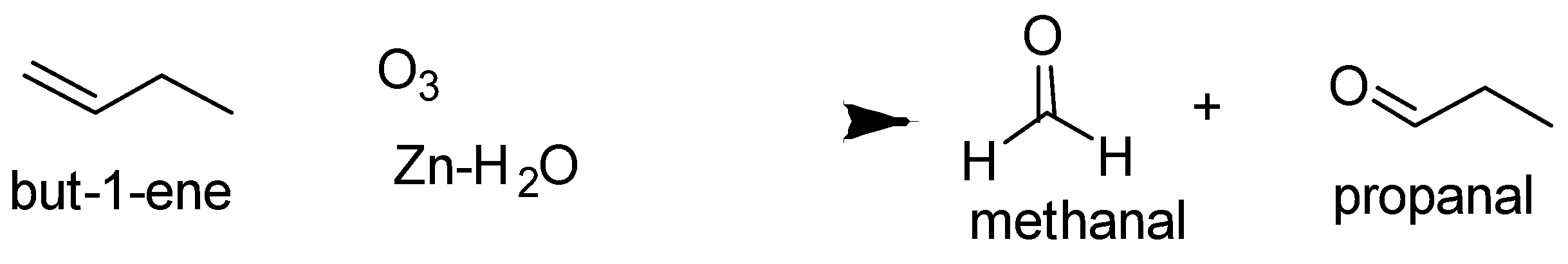
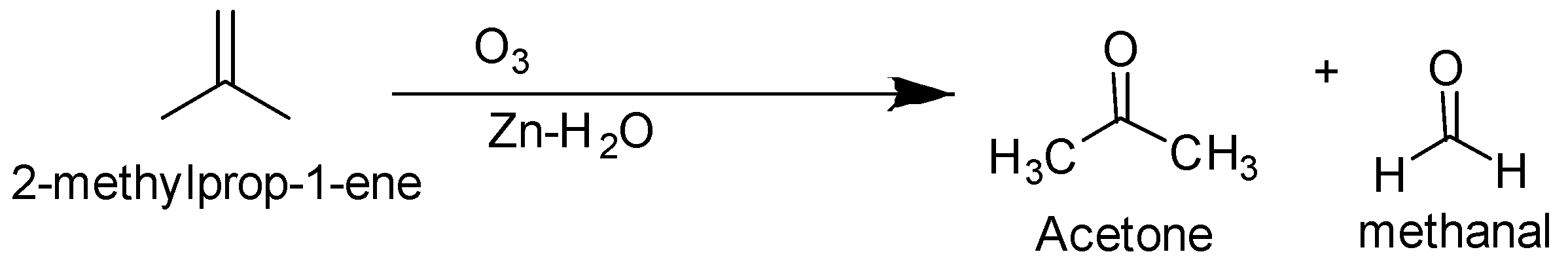
So the alkene is but −2− ene .
When alcohol undergoes acidic hydrolysis dehydration of alcohol takes place and alkene is formed.
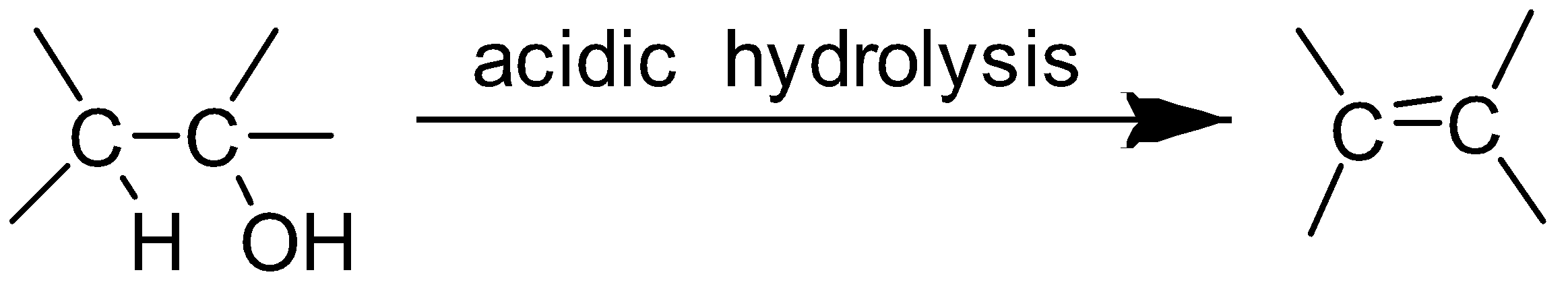
The alcohol which on acidic hydrolysis gives but −2− ene is butan −2− ol.
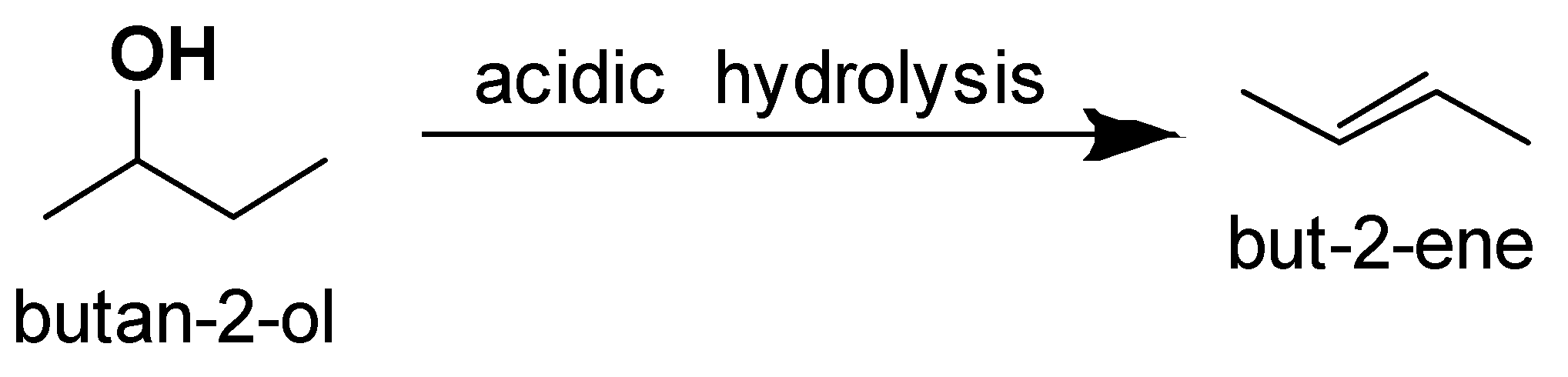
So the correct option is B.
Note: The reagents required for ozonolysis are O3andZn−H2O .
-There is a trick for identifying the ozonolysis reaction product. Cleave the carbon-carbon bond and convert the end carbon atoms of the double bond into carbonyl groups. Zinc prevents the molecule from further forming a bond with oxygen molecules. It prevents further oxidation.
-Dehydration is the process of removal of water molecules.
