Question
Question: An alcohol of molecular formula \[{C_5}{H_{14}}OH\] on dehydration gives an alkene, which on oxidati...
An alcohol of molecular formula C5H14OH on dehydration gives an alkene, which on oxidation yields a mixture of ketone and an acid. The alcohol is:
A. CH3CH2CH(OH)CH2CH3
B. CH2−OH∣CHCH2CH2CH3
C. (CH3)2CHCH(OH)CH3
D. (CH3)3CCH2OH
Solution
The alcohol C5H14OH undergoes dehydration using sulphuric acid to produce alkene with molecular formula (CH3)2CCHCH3 which on oxidation with potassium permanganate gives a carboxylic acid and a ketone.
Complete step by step answer: Given,
The molecular formula of alcohol is C5H14OH.
It is given that the alcohol on dehydration gives alkene which further on oxidation gives mixture of ketone and an acid as the main product.
Dehydration is the reaction used for the conversion reactant into product by removing water and using a dehydrating reagent. This reaction is the reverse of the hydration reaction where a water molecule is added to the reactant to form the product.
In oxidation reaction, the carbon atoms gain electrons from electronegative atoms mostly oxygen.
The reaction of alcohol with sulphuric acid and further oxidizing with potassium permanganate is shown below
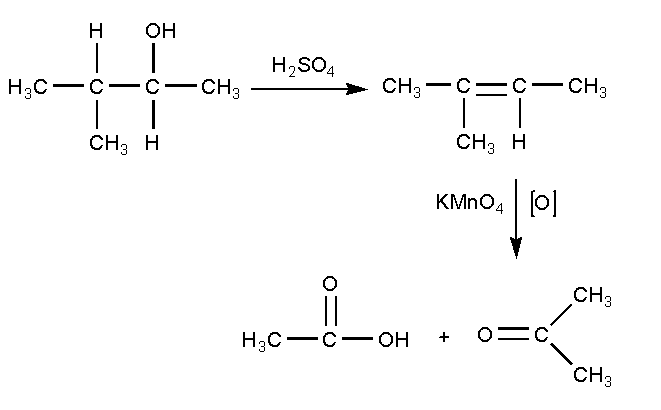
In this reaction, first 3-methylbutan-2-ol reacts with sulphuric acid H2SO4 where the lone pairs present on the oxygen of hydroxyl group abstract the hydrogen of sulphuric acid and bond will shift to oxygen forming an anion. Further the water molecule will be removed and the bond will shift to form an alkene product. The product formed is 2-methyl-but-2-ene. The compound 2-methyl-but-2-ene is oxidized using oxidizing agent potassium permanganate to give a carboxylic acid and a ketone. The carboxylic acid product is ethanoic acid and the ketone product is propan-2-one.
Therefore, the alcohol with molecular weight C5H14OH is (CH3)2CHCH(OH)CH3.
Therefore, the correct option is C.
Note:
Apart from sulphuric acid, the other dehydrating agent is concentrated phosphoric acid, hot aluminium oxide. The other oxidizing agent is hydrogen peroxide, ozone, nitric acid.
