Question
Question: An alcohol A \(({C_{10}}{H_{18}}O)\) is converted into a mixture of alkenes B and C on being heated ...
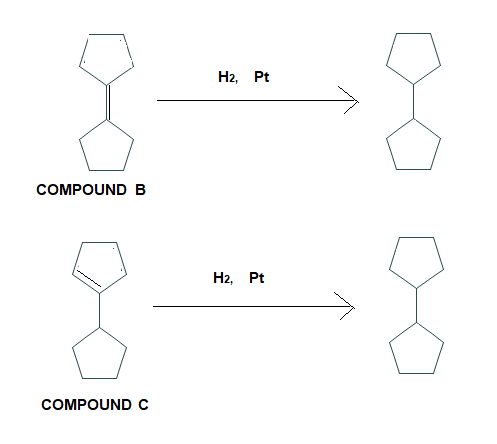
An alcohol A (C10H18O) is converted into a mixture of alkenes B and C on being heated with potassium hydrogen sulphate (KHSO4) . Catalytic hydrogenation of B and C yield the same product. Assuming the dehydration of alcohol proceeds without rearrangement, deduce the structure of alcohol A and alkenes C .
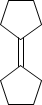
COMPOUND-B
Solution
Potassium hydrogen sulphate is used as a dehydrating agent. Dehydration means removal of water. Hydrogenation is the addition of hydrogen molecules. Such processes generally require catalysts.
Complete step by step answer:
Potassium hydrogen sulphate is a dehydrating agent. The elimination of water molecules from a compound is called dehydration. When alcohols are treated with dehydrating agents, they give alkene as a product. This reaction is also called as beta− elimination of alcohols.
In the dehydration reaction of alcohol, the −OH group from the alpha carbon and hydrogen from the beta carbon is lost as the water molecule. If there are more than one type of beta hydrogen , then more than one type of product is possible.
The major product of dehydration of alcohol is given by Saytzeff’s rule. In the elimination reaction of alcohols, the more substituted alkene is formed as the major product. The other minor product is the less substituted alkene.
Now alcohol A gives alkene B and C. The structure of B is given . Now we can predict the structure of C. it might be-
COMPOUND-C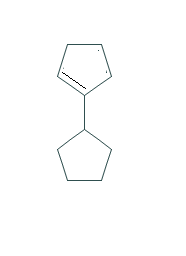
As we can see that the only possible alkene could be the above alkene. So we can say that compound B is major alkene and compound C is minor alkene.
Catalytic hydrogenation is the addition of hydrogen across the unsaturated site in the presence of a catalyst. The catalysts used are nickel, palladium or platinum.
Compound B and Compound C on catalytic hydrogenation will give the same product.