Question
Question: Amongst the following, the most stable complex is: A. \({[Fe({H_2}O){}_6]^{3 + }}\) B. \({[Fe{(...
Amongst the following, the most stable complex is:
A. [Fe(H2O)6]3+
B. [Fe(NH3)6]3+
C. [Fe(C2O4)3]3−
D. [FeCl6]3−
Solution
A ligand is an ion or molecule, which donates a pair of electrons to the central metal atom or ion to form a coordination complex. Lewis base which donates two lone pairs of electrons to the central metal atom is known as a bidentate ligand. They are often referred to as “chelating ligands”.
Complete step by step answer:
The bidentate ligands are known as chelating ligands and the complex which contains the chelating ligands is called Chelates.
Further, the complexes that contain the chelate rings are more stable than the complexes without the rings and this effect is known as chelate effect.
Now, among the given options, the most stable compound is [Fe(C2O4)3]3−.
However, in each of the given complexes, Fe is in +3 oxidation state but in case of [Fe(C2O4)3]3− , [C2O4]]2− is a bidentate chelating ligand and thus it forms rings and hence, becomes the most stable complex. The structure of the complex is as shown:
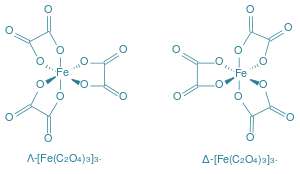
Hence. Option C is correct.
Note:
Ligands with more than one potential donor atoms are known as ambidentate ligands. For example, thiocyanate ion (NCS−) which can bind to the central metal atom or ion with either nitrogen or sulfur atoms. Moreover, bridging ligands are the one that are bound to more than one metal atom.
