Question
Question: Amongst the following the most basic compound is: A. p-nitroaniline B. Acetanilide C. Aniline ...
Amongst the following the most basic compound is:
A. p-nitroaniline
B. Acetanilide
C. Aniline
D. Benzylamine
Solution
Hint: Amines are bases due to the presence of a lone pair of electrons on the nitrogen atom. The tendency of nitrogen atoms to share these electrons with acid is responsible for the basic strength of amines.
Complete step-by-step answer:
The structure of the compounds are given below;
As we know that the N containing compounds depends on the availability of lone pairs of electrons on N.
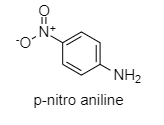
p-nitroaniline: Nitro group at p-position pulls the lone pair of electrons of N more strongly than aniline. Hence, lone pairs of electrons are not available.
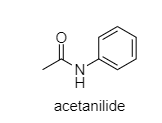
Acetanilide: In this compound, there are two side pulling of lone pairs of electrons of N. One side by benzene ring and the other side by C=O group. So, the lone pair of electrons on N are not available.
Aniline: Lone pairs of electrons on N are involved in resonance with an aromatic benzene ring. Hence, lone pairs are not available easily.
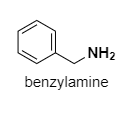
Benzylamine: Here, the lone pairs are not in conjugation with the benzene right. It is very much like a primary amine and lone pair of electrons on N are easily available.
Hence, the most basic compound is Benzylamine and the correct option is D.
Note: The possibility to make a mistake is that you may choose C. But benzylamine is more basic than aniline as the benzyl group is an electron-donating group due to the +I effect. So, the electron density increases on N of NH2.
