Question
Question: Amongst the above conformations of 3 – Aminopropanal (P, Q, R, S), one of them is more stable. This ...
Amongst the above conformations of 3 – Aminopropanal (P, Q, R, S), one of them is more stable. This can be attributed due to:
I)H – bonding in the conformer
II)gauche conformation in the conformer
III)anti – conformation in the conformer
IV)larger groups being separated by maximum distance in the conformer
The correct option is:
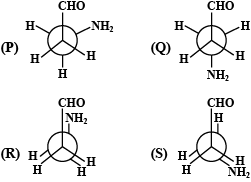
A.II and IV
B.I and II
C.III and IV
D.I and IV
Solution
In the (P) conformation, the aldehyde group and the amino group are adjacent to each other and they form intramolecular hydrogen bonds. This can be attributed due to H − bonding in the conformer and the gauche conformation in the conformer.
Complete step by step answer:
In the conformation, (P), the hydrogen atom of the aldehyde group points towards the nitrogen atom of the amino group, and a stationary point with a horizontal tangent plane and the C-H···N interaction turns out to be electronically repulsive (the C−H bond length is significantly shorter than in all other conformers, and the C−H vibration frequency is more than 100c−1 above the average). However, there is enough electrostatic attraction between the positively charged hydrogen atom and the negatively charged nitrogen atom to overcome this repulsion i.e. form intermolecular hydrogen bonds.
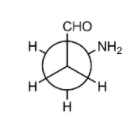
So, as the aldehyde group and the amino group are adjacent to each other. They form an intramolecular hydrogen bonds and this can be attributed due to the following reasons:
(I) H−bonding in the conformer
(II) gauche conformation in the conformer
Therefore, the correct answer is option (B).
Note: 3 – aminopropanal is a propanal that has an amino substituent at the 3 – position It is an omega – amino aldehyde and a member of propanals. Its molecular formula is C3H7NO. It is a conjugate base of a 3 – aminopropanal. Its molecular weight is 73.09molg. 3 – aminopropanal is so unstable because it has two functional groups that may react with many different substrates and for each reaction there are conformers, which are of advantage for an approach to the respective substrate, and others that are of disadvantage.
