Question
Question: Amongst \[N{O_3}^ - ,{\text{ }}As{O_3}^{3 - },{\text{ }}C{O_3}^{2 - },{\text{ }}Cl{O_3}^ - ,{\text{ ...
Amongst NO3−, AsO33−, CO32−, ClO3−, SO32−andBO33−, the non-planar species are :
{A:N{O_3}^ - ,{\text{ }}C{O_3}^{2 - },{\text{ }}and{\text{ }}B{O_3}^{3 - }} \\\ {B:{\text{ }}S{O_3}^{2 - },{\text{ }}Cl{O_3}^ - ,{\text{ }}and{\text{ }}As{O_3}^{3 - }} \\\ {C:{\text{ }}C{O_2}^{3 - },{\text{ }}S{O_3}^{2 - }{,^{}}andB{O_3}^{3 - }} \\\ {D:{\text{ }}As{O_3}^{3 - },{\text{ }}C{O_3}^{2 - },{\text{ }}and{\text{ }}S{O_3}^{2 - }} \end{array}$$Solution
A planar molecule refers to a molecule with all its atoms lying in one two-dimensional plane. Molecules having linear, square or triangular geometries are said to be planar molecules. In other cases, molecules may form three-dimensional shapes, like tetrahedrons, bipyramids or octahedrons.
Complete step by step answer:
First of all you have to find the hybridization of molecules. You can remember following rules to decide whether a molecule is planar or non-planar:
- The molecule is not considered to be planar If there exists a sp3 hybridized carbon (or nitrogen).
- The molecule is considered to be planar if there is no sp3hybridized carbon (or nitrogen), but there is one sp2 h hybridized carbon or nitrogen atom.
- The molecule is not considered to be planar if there is no sp3 hybridized atom but there are two sp2 hybridized atoms which are separated by an even number of double bonds.
We can say that the molecule is not planar if there exists either a sp3 hybridized atom or two sp2 hybridized atoms that are being separated by even numbers of double bonds. In other cases, its structure is planar. For the present scenario, consider the following table:
| Species | Hybridization | Geometry | No. of lone pairs |
|---|---|---|---|
| NO3- | sp2 | 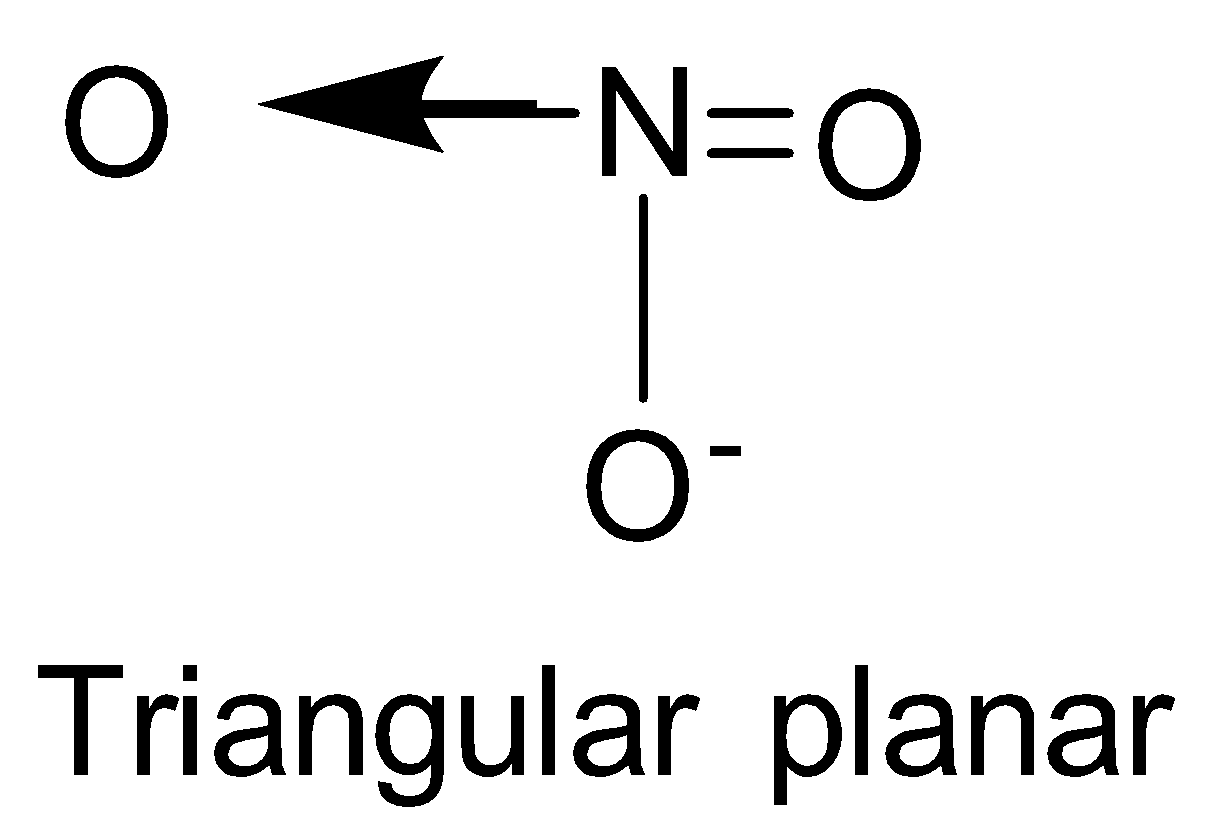 | 0 |
| AsO33- | sp3 | 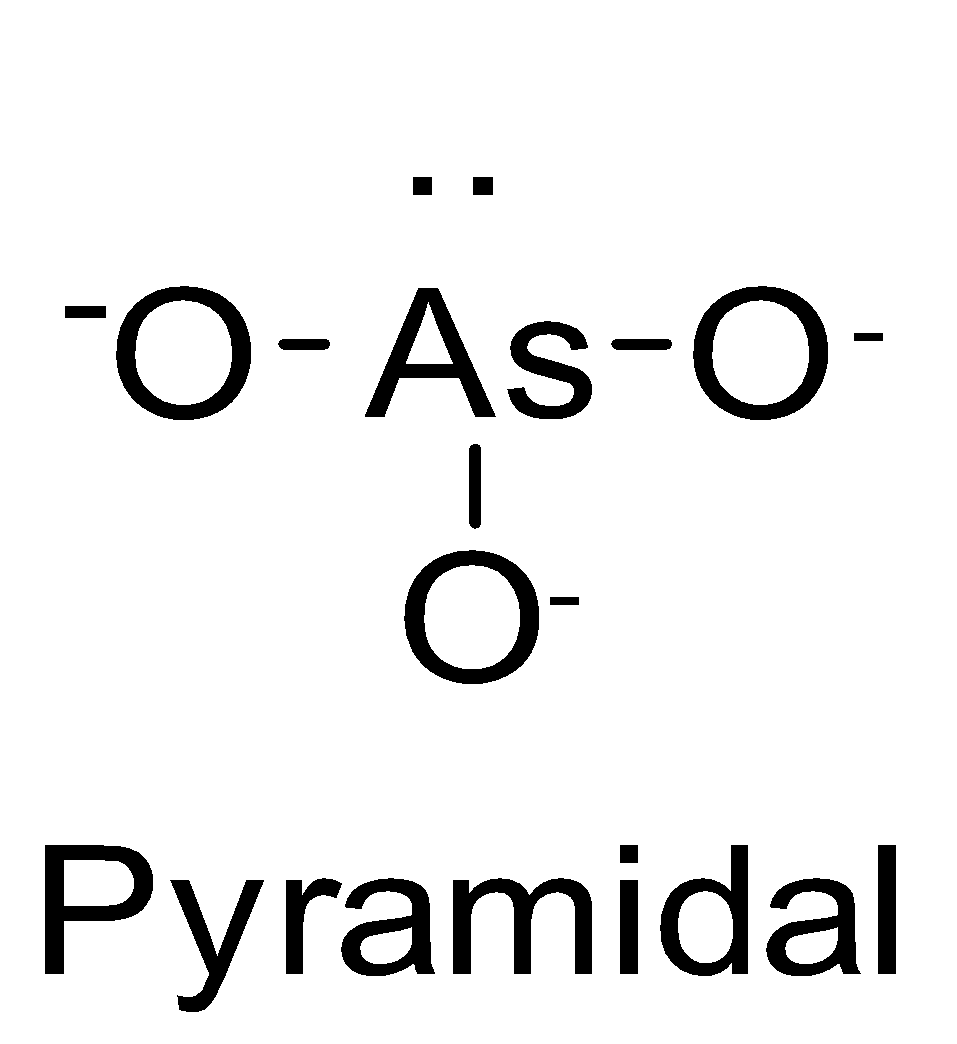 | 1 |
| CO32- | sp2 | 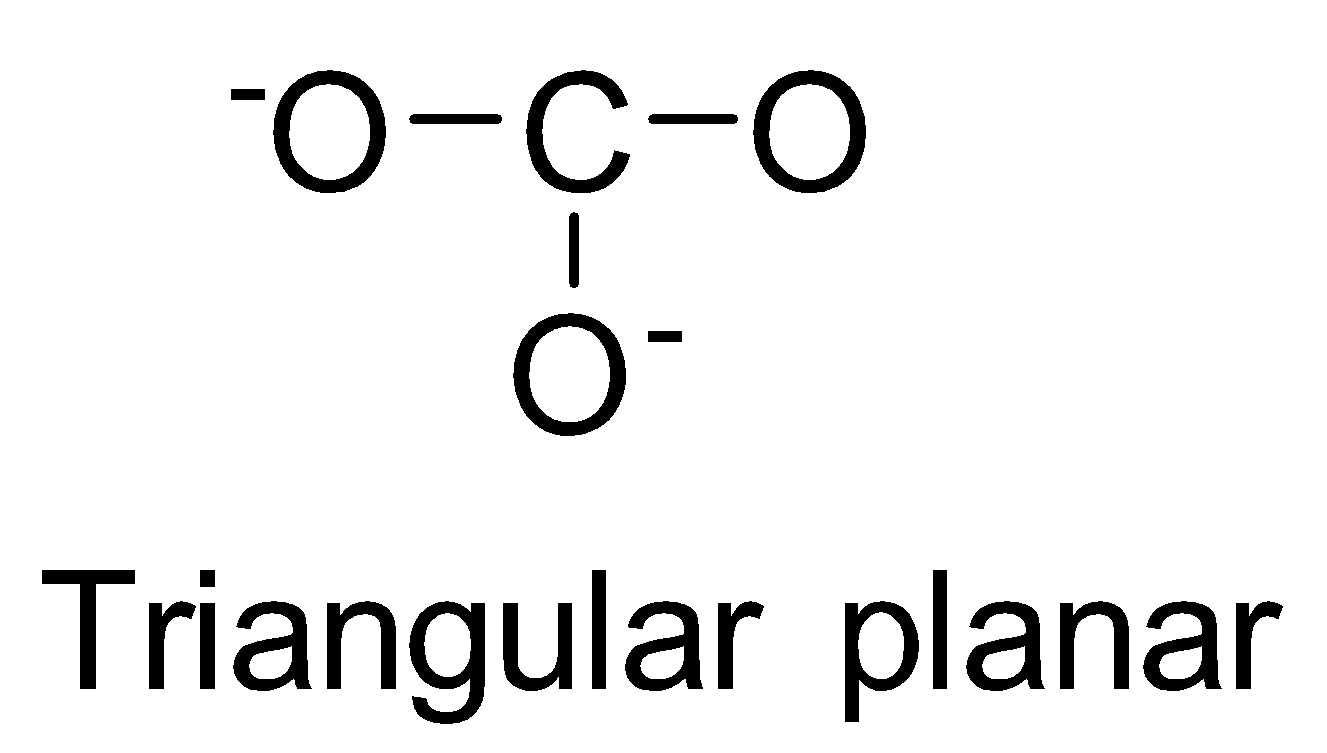 | 0 |
| ClO3- | sp3 | 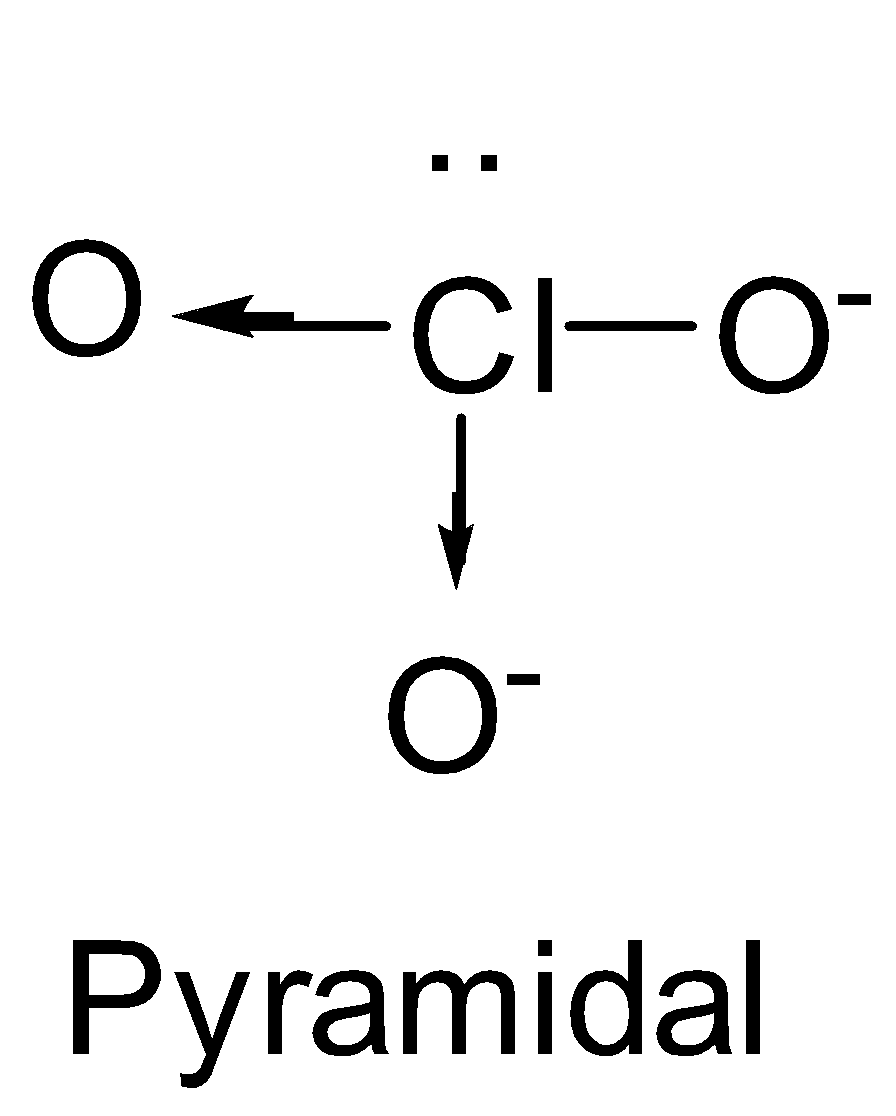 | 1 |
| SO32- | sp3 | 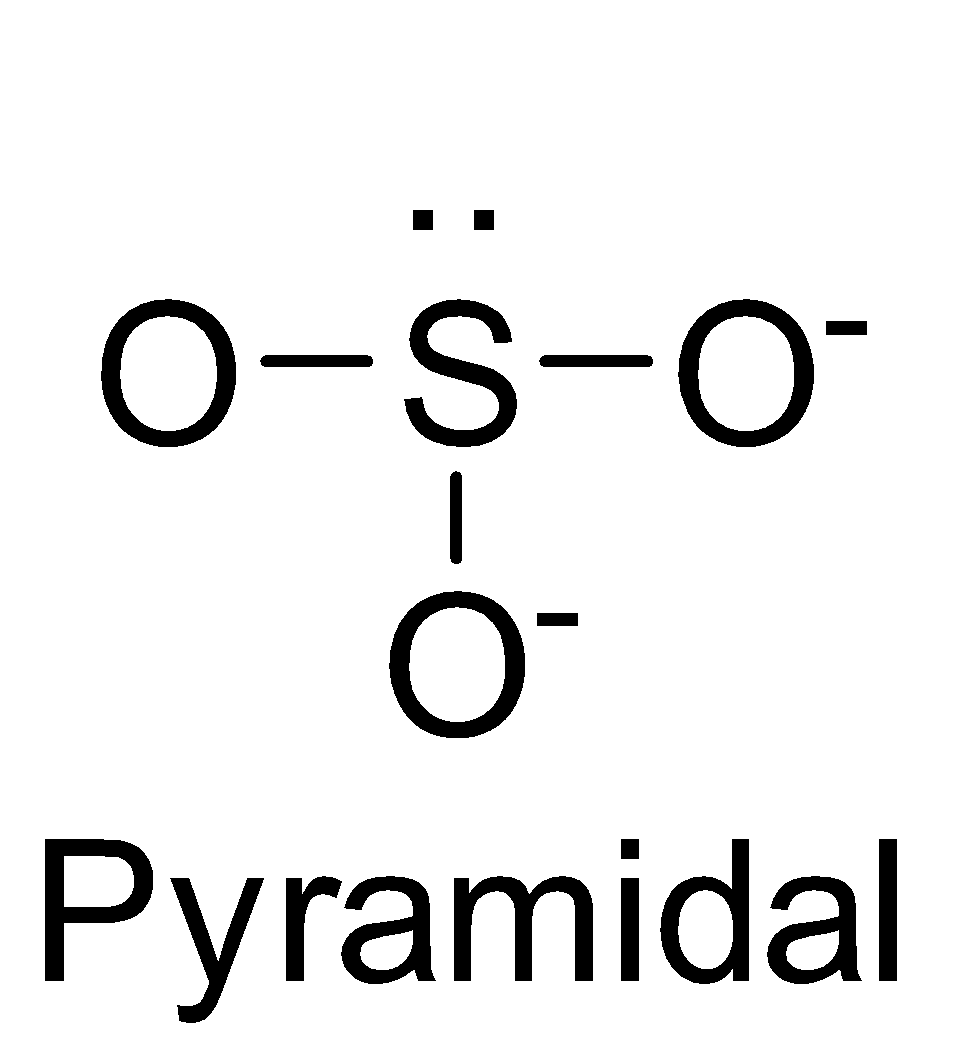 | 1 |
| BO33- | sp2 | 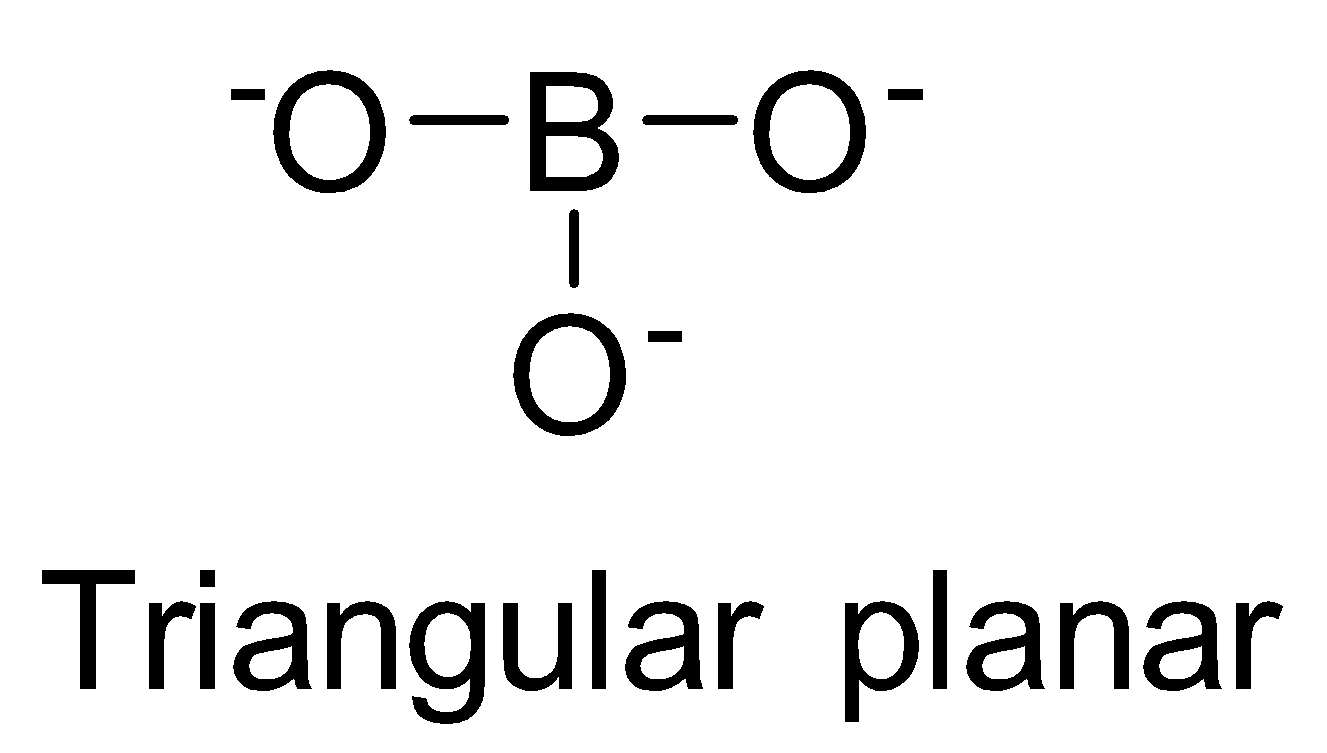 | 0 |
NO3−, CO32−and BO33− possess sp2 hybridisation and thus, are planar species.
Hence, the correct answer is Option B.
Note:
You can remember that in order to find the hybridization of a molecule, count the valence electrons of each atom in a compound (fact to be noted is that count valence electron of hydrogen = 7). Add them all and divide the number by 8. Quotient represents bond pair while 2remainder represents lone pair. Add quotient and 2remainder and assume it as x. If (i) x is 3, molecule is planar with sp2 hybridisation, (ii) x is 2, molecule is linear with sp hybridization and x is 4, molecule is non-planar with sp3 hybridization.
