Question
Question: Among these cations, which of the following orders is correct for their no- bond- resonance energy? ...
Among these cations, which of the following orders is correct for their no- bond- resonance energy?
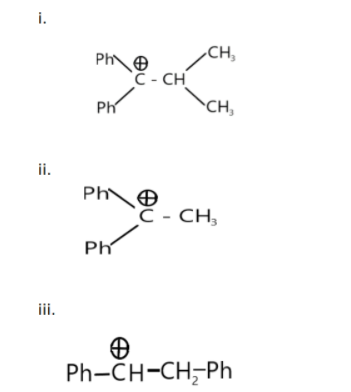
a. i > ii > iii
b. iii > i > ii
c. iii > ii > i
d. ii > iii > i
Solution
When there is a positive charge on carbon the compound is generally called carbocation, all the structure has 1 carbocation, no – bond – resonance is called hyperconjugation, where the sigma or lone pair electrons move to the adjacent pi orbital.
Complete Step by step answer: First, let’s understand the role of hyperconjugation in a molecule?
Hyperconjugation or no – bond – resonance brings stability to the molecule, the more the extent of hyperconjugation the more will be the stability of the corresponding molecule.
Hyperconjugation is directly proportional to the number of α hydrogen present in the given molecule. Hence, the greater the number of α hydrogen greater will be the hyperconjugation or non-bond- resonance energy.
The carbon adjacent to the function group is called α carbon, and the hydrogen attached to the α carbon is called α hydrogen.
Therefore, to choose the correct option we have to count the α hydrogen present in each molecule.
The maximum number of α hydrogen is present in molecule ‘ii’ and followed by 2 and 1 in molecule ‘iii and i’ respectively. The order of hyperconjugation can be written as ii > iii > i
Hence, the correct answer is option (d) i.e., ii > iii > i.
Note: The phenyl group (−C6H5) has 5 hydrogens but these hydrogen are not α hydrogen as the α carbon bonded with carbocation has no hydrogen in it. The functional group is usually alkene, but here one hydrogen from one of the alkene carbon is removed, leaving a + ve charge behind. Hence, we will consider the carbocation (C⊕) as the functional group.
