Question
Question: Among the three molecules, \(Xe{F_4}\), \(S{F_4}\)and \(Si{F_4}\), which has/ have tetrahedral struc...
Among the three molecules, XeF4, SF4and SiF4, which has/ have tetrahedral structure?
A) All three
B) SiF4 and SF4
C) Only SiF4
D) Only SF4
Solution
To answer this question you must recall the VSEPR theory. The Valence shell electron pair repulsion theory proposes that the hybridized orbitals in an atom arrange themselves in such a way so as to minimize the repulsion between them, hence determining the geometry of a molecule on the basis of its hybridization.
Complete step by step solution:
First we consider, XeF4.
We know that Xenon has 8 valence electrons. Since it forms four bonds with fluorine, thus, we can conclude that 2 electrons from the 5p orbital are excited to the empty 5d orbital. The atom undergoes an sp3d2 hybridisation. The geometry of the molecule is octahedral. The hybrid orbitals form four bond pairs and two lone pairs. Thus, the structure is square planar.
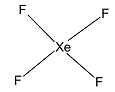
Now, we consider SF4.
Sulphur has 6 valence electrons out of which 2 are unpaired. One electron from the 3p orbital is excited to 3d orbital and the atom undergoes sp3d hybridization. Geometry of molecules is trigonal bipyramidal but due to the presence of a lone pair the structure is see-saw shaped.
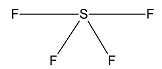
Considering SiF4
Silicon has 4 valence electrons. One valence electron from 3s is excited to 3p. The atom undergoes sp3 hybridisation. All four hybrid orbitals form bond pairs and the structure is tetrahedral.
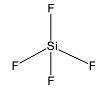
Thus, the correct answer is C.
Note:
The concept of mixing of atomic orbitals in order to form new hybrid orbitals that possess different shapes and energies as compared to the original parent atomic orbitals is known as hybridisation. Hybrid orbitals are suitable to form chemical bonds of equal energies. Also hybridization of orbitals leads to the formation of more stable compounds because hybrid orbitals have lower energy than the unhybrid orbitals.
