Question
Question: Among the naturally occurring carbohydrates, furanose ring is found in the: A.glucose unit of cane...
Among the naturally occurring carbohydrates, furanose ring is found in the:
A.glucose unit of cane sugar
B.glucose unit of cellulose
C.fructose unit of cane sugar
D.galactose unit of lactose
Solution
To answer this question, you should recall the concept of furanose sugars. Furanose is a term which designates carbohydrates with a five-membered ring (cyclic) structure, consisting of 4 carbon atoms and single oxygen. It is called furanose because of its structural similarity to furan.
Complete Step by step solution:
We know that the different structures of carbohydrates that result from a keto form are:
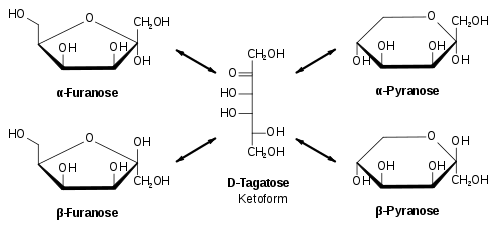
We know that the reducing carbohydrates possess anomeric carbon which acts as a chiral centre for the cyclization of the molecules. The cyclization of the carbohydrate molecules forms two different Structural isomers –
- Pyranose
- furanose
The same rule applies to fructose as it also exists in a cyclic form which is known as furanose with an analogy to the compound furan which is a five-membered cyclic compound with one oxygen and four carbon atoms. The structure of furanose can be drawn as:
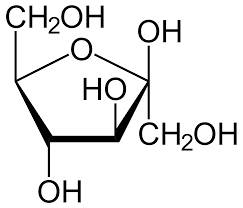
Therefore, we can conclude that the correct answer to this question is option C.
Note: You should know that if you have seen drawings of sugars before, you might not have noticed the carbonyl. The reason is that carbonyl is usually "masked" as a hemiacetal structure. The hemiacetal structure is generated when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. We can see from the structure of fructose that the keto group is present on the second carbon. Now two possibilities for cyclization exist: If attacked by the - CH2OH on the 6th carbon it will generate pyranose (6-membered) ring and second that, attacked by the - CH(OH) - group on the 5th carbon it will result in a furanose. In general, we can conclude that pyranose rings are more stable than furanose rings, because there are more conformational isomeric structures available for pyranose vs. furanose.
