Question
Question: Among the given reactions which is faster and why? I. \(Me-CH(Cl)-Me\underrightarrow{^{ alc.KOH }}...
Among the given reactions which is faster and why?
I. Me−CH(Cl)−Mealc.KOHCH3−CH=CH2
II. CH3−CH2−CH2−Clalc.KOHCH3−CH=CH2
Solution
Hint: The answer of the question lies in the mechanism of this elimination reaction and the stability of carbocation. We know the alkyl halide and alcoholic KOH proceeds through E1 elimination reaction and the order of stability of carbocation is 3o>2o>1o.
Complete step by step solution:
As we know, Alkenes are generally prepared through β elimination reactions, in which two atoms on adjacent carbon atoms are removed, resulting in the formation of a double bond. This is also called dehydration reaction or elimination reaction.
If a haloalkane is heated with concentrated alcoholic potassium hydroxide the major product formed is an alkene due to the elimination of hydrogen halide. This is called β elimination or dehydrohalogenation.
Chloroethanealc.KOHEthene
C2H5Clalc.KOHCH2=CH2
In this reaction, the OH− ion acts as a base and removes protons from the molecule.
For better understanding, the mechanism of elimination reaction are mentioned in three steps-
1. Proton removal
2. C=C bond is formed
3. There is a breakage in the bond of leaving group
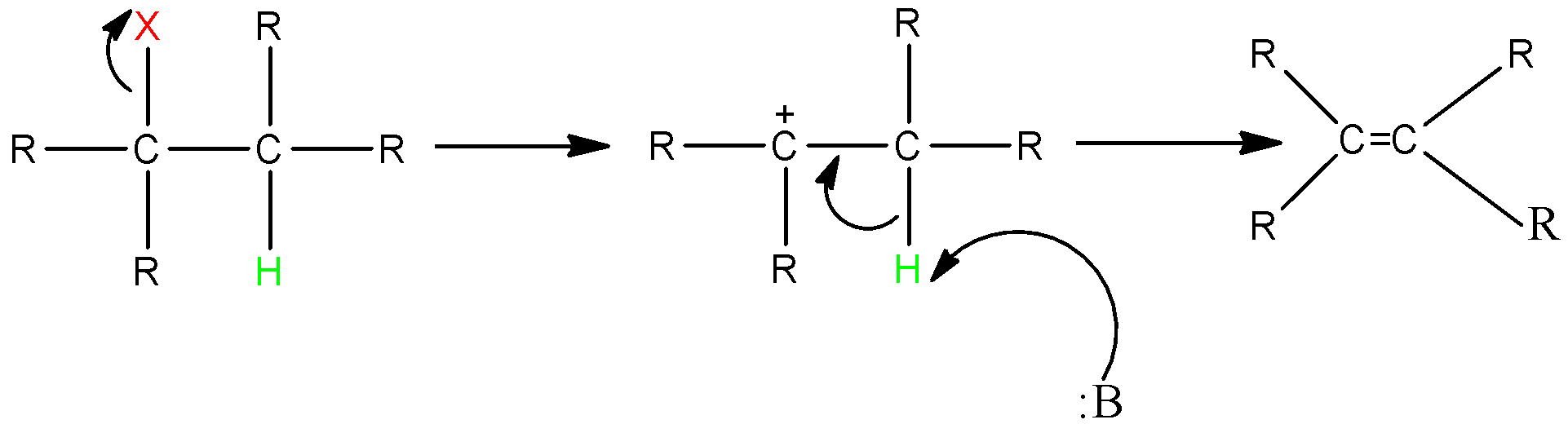
E1 Reaction:
In the E1 mechanism which is also known as unimolecular elimination, there are usually two steps involved-ionization and deprotonation.
During ionization, there is a formation of carbocation as an intermediate. In deprotonation, a proton is lost by the carbocation.
This happens in the presence of a base which further leads to the formation of a pi-bond in the molecule.
In E1, the reaction rate is also proportional to the concentration of the substance to be transformed.
It exhibits first order kinetics.
As per the discussion, option (i) is faster as there is formation of 2o carbocation which is stable than 1o carbocation.
Note: It should be noted that if the structure of alkyl halide is such that it can undergo elimination in two different ways, then the more highly substituted alkene (i.e. having lesser number of hydrogen atoms on the doubly bonded carbon atoms) is the major product of elimination. This is known as Saytzeff’s rule. It is also pronounced as Zaitsev rule.
