Question
Question: Among the given organic compounds, the total number of aromatic compounds is...
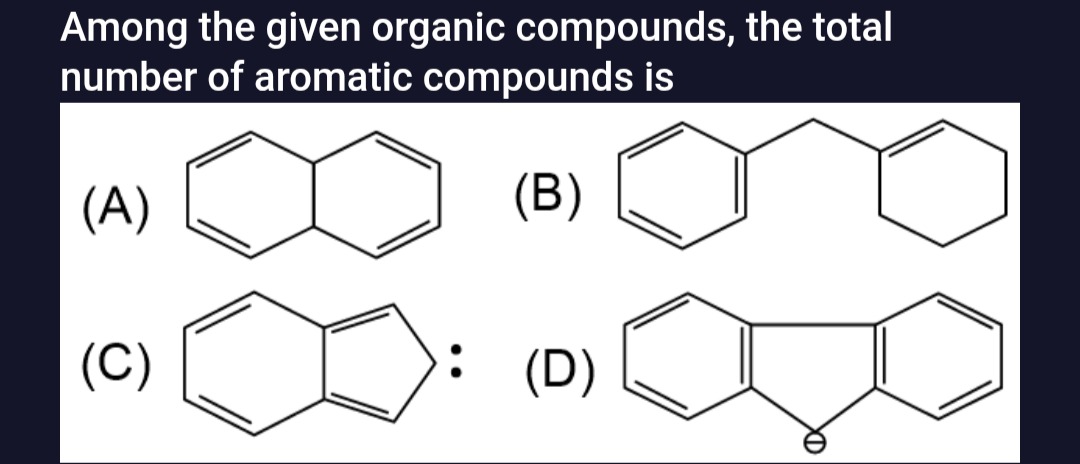
Among the given organic compounds, the total number of aromatic compounds is
1
2
3
4
1
Solution
Here's the analysis of each compound for aromaticity:
Compound (A): This compound is a fused bicyclic system. It has two fused six-membered rings. There are four double bonds shown in the conjugated system.
- Cyclic: Yes, it is a cyclic compound.
- Planar: The fused ring system may not be planar due to the presence of the bridgehead carbons and the arrangement of double bonds. However, for aromaticity, planarity is a key requirement.
- Conjugated: The double bonds are conjugated.
- Hückel's Rule: The total number of pi electrons is 4×2=8. For aromaticity, the number of pi electrons must be of the form (4n+2). Since 8=4n+2 for any integer n, it does not satisfy Hückel's rule. If it were planar, it would be anti-aromatic. However, due to likely non-planarity, it is considered non-aromatic.
Compound (B): This compound consists of a benzene ring attached to a cyclohexene ring via a methylene group. The benzene ring is aromatic. However, the cyclohexene ring is not. The entire molecule is not aromatic as a whole. Generally, when asked about aromatic compounds, the entire molecule's aromaticity is considered. Thus, compound (B) is not an aromatic compound.
Compound (C): This compound is a fused bicyclic system consisting of a six-membered benzene ring fused to a five-membered ring. The five-membered ring contains a double bond and a lone pair of electrons (represented by two dots) on a carbon atom. This structure represents the Inden-1-ide anion.
- Cyclic: Yes, it is a cyclic compound.
- Planar: The benzene ring is planar, and the five-membered ring with delocalized pi electrons can achieve planarity.
- Conjugated: The system is conjugated. The benzene ring contributes 6 pi electrons. The five-membered ring has a double bond (2 pi electrons) and a lone pair on a carbon atom (2 electrons) that participates in the pi system.
- Hückel's Rule: The total number of pi electrons is 6 (from benzene) +2 (from double bond) +2 (from lone pair) =10. This fits Hückel's rule, as 10=4n+2 with n=2. Therefore, compound (C) is aromatic.
Compound (D): This compound is a fused bicyclic system containing a benzene ring and a five-membered ring with a double bond and a carbocation.
- Cyclic: Yes, it is a cyclic compound.
- Planar: The benzene ring is planar. The presence of a carbocation and a double bond in the five-membered ring suggests it could be planar.
- Conjugated: The system is conjugated. The benzene ring contributes 6 pi electrons. The double bond contributes 2 pi electrons. The carbocation contributes 0 pi electrons to the pi system.
- Hückel's Rule: The total number of pi electrons is 6 (from benzene) +2 (from double bond) =8. This does not fit Hückel's rule (4n+2). If planar, it would be anti-aromatic. Thus, compound (D) is not aromatic.
Based on the analysis, only compound (C) is aromatic.
The total number of aromatic compounds among the given options is 1.
