Question
Question: Among the following which is / are more stable than $CH_2=CH-CH_2^{\ominus}$...
Among the following which is / are more stable than CH2=CH−CH2⊖
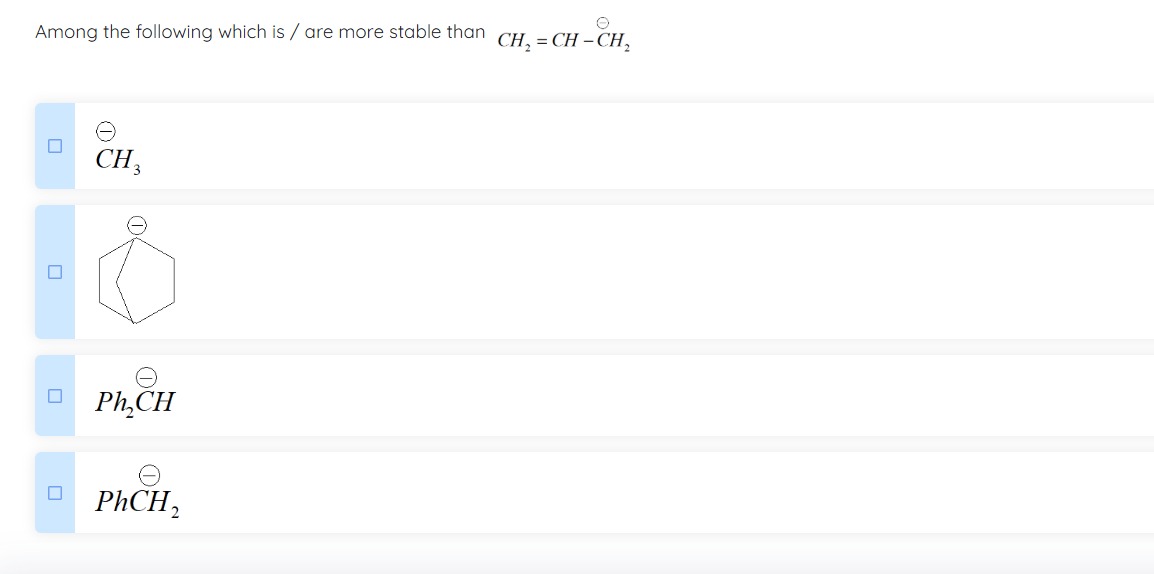
A
CH3⊖
B
Cyclohexadienyl carbanion
C
Ph2CH⊖
D
PhCH2⊖
Answer
Ph2CH⊖, PhCH2⊖, Cyclohexadienyl carbanion
Explanation
Solution
The stability of carbanions is determined by factors like resonance, inductive effects, and hybridization. The allyl carbanion (CH2=CH−CH2⊖) is stabilized by resonance delocalization over two carbons.
- CH3⊖: Less stable due to lack of resonance and electron-donating alkyl groups.
- Cyclohexadienyl carbanion: Resonance stabilization within the ring system makes it more stable than allyl.
- Ph2CH⊖: Highly stable due to extensive resonance delocalization into two phenyl rings.
- PhCH2⊖: More stable than allyl due to resonance delocalization into one phenyl ring.
Thus, Ph2CH⊖, PhCH2⊖, and the cyclohexadienyl carbanion are more stable than CH2=CH−CH2⊖.
