Question
Question: Among the following, the number of aromatic compound(s) having only $sp^2$ hybridized atoms is www....
Among the following, the number of aromatic compound(s) having only sp2 hybridized atoms is
AYJR Advanced (Paper 1)
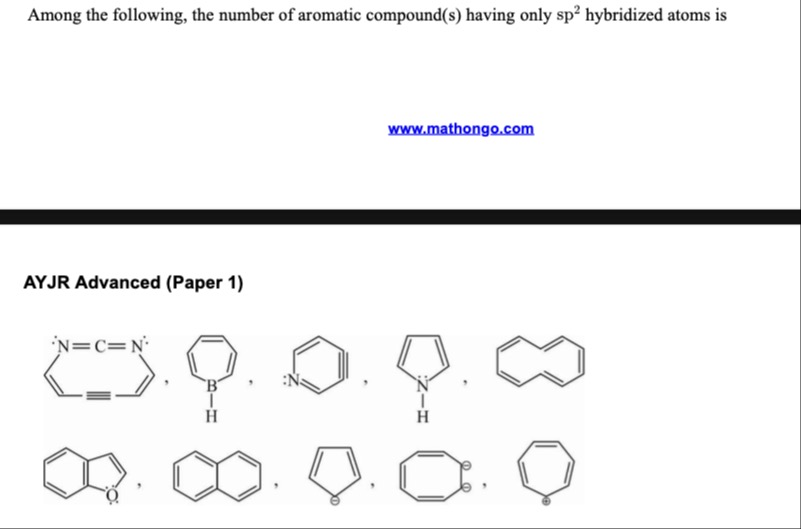
10
Solution
The question asks for the number of aromatic compounds among the given structures that have only sp2 hybridized atoms.
Let's analyze each compound:
-
[18]Annulene: This is a cyclic conjugated hydrocarbon with 18 carbon atoms and 9 double bonds. It has 18 pi electrons. According to Huckel's rule, 4n+2=18 when n=4. [18]Annulene is aromatic. In [18]annulene, all carbon atoms are sp2 hybridized as they are part of a conjugated system of double and single bonds, forming three sigma bonds and one pi bond. Thus, this compound is aromatic and has only sp2 hybridized atoms.
-
Borabenzene: This is a cyclic compound with 5 carbon atoms and 1 boron atom, with alternating double and single bonds. It has 3 double bonds, contributing 6 pi electrons. According to Huckel's rule, 4n+2=6 when n=1. Borabenzene is aromatic. The carbon atoms are sp2 hybridized. The boron atom is bonded to two carbon atoms and one hydrogen atom, forming three sigma bonds. Thus, the boron atom is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Pyridine: This is a cyclic compound with 5 carbon atoms and 1 nitrogen atom, with alternating double and single bonds. It has 3 double bonds, contributing 6 pi electrons. The nitrogen atom has one lone pair which is in an sp2 orbital and is not part of the pi system. The pi system consists of the 6 electrons from the double bonds. According to Huckel's rule, 4n+2=6 when n=1. Pyridine is aromatic. The carbon atoms are sp2 hybridized. The nitrogen atom forms two sigma bonds with carbon atoms and has one lone pair. The steric number is 2+1=3. Thus, the nitrogen atom is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Pyrrole: This is a cyclic compound with 4 carbon atoms and 1 nitrogen atom, with two double bonds. The nitrogen atom is bonded to a hydrogen atom. It has two double bonds (4 pi electrons) and one lone pair on nitrogen (2 electrons) which is in a p orbital and participates in the pi system. Total pi electrons = 4+2=6. According to Huckel's rule, 4n+2=6 when n=1. Pyrrole is aromatic. The carbon atoms are sp2 hybridized. The nitrogen atom forms three sigma bonds (to two carbons and one hydrogen) and has one lone pair in a p orbital. Thus, the nitrogen atom is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Naphthalene: This is a fused bicyclic compound with 10 carbon atoms. It has a conjugated system of double bonds. It has 5 double bonds, contributing 10 pi electrons. According to Huckel's rule, 4n+2=10 when n=2. Naphthalene is aromatic. All carbon atoms in naphthalene are sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Furan: This is a cyclic compound with 4 carbon atoms and 1 oxygen atom, with two double bonds. The oxygen atom has two lone pairs. It has two double bonds (4 pi electrons) and one lone pair on oxygen (2 electrons) which is in a p orbital and participates in the pi system. Total pi electrons = 4+2=6. According to Huckel's rule, 4n+2=6 when n=1. Furan is aromatic. The carbon atoms are sp2 hybridized. The oxygen atom forms two sigma bonds with carbon atoms and has two lone pairs. It is sp2 hybridized, with one lone pair in a p orbital and the other in an sp2 orbital. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Anthracene: This is a fused tricyclic compound with 14 carbon atoms. It has a conjugated system of double bonds. It has 7 double bonds, contributing 14 pi electrons. According to Huckel's rule, 4n+2=14 when n=3. Anthracene is aromatic. All carbon atoms in anthracene are sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Cyclopentadienyl anion: This is a cyclic compound with 5 carbon atoms and a negative charge. It has two double bonds (4 pi electrons) and a negative charge (lone pair, 2 electrons) on one carbon atom which participates in the pi system. Total pi electrons = 4+2=6. According to Huckel's rule, 4n+2=6 when n=1. Cyclopentadienyl anion is aromatic. The carbon atoms in the double bonds are sp2 hybridized. The carbon atom with the negative charge forms two sigma bonds and has a lone pair in a p orbital. Thus, it is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Cyclooctatetraene dianion: This is a cyclic compound with 8 carbon atoms and a 2- negative charge. Neutral cyclooctatetraene is antiaromatic and non-planar. However, the dianion is planar and conjugated. It has 4 double bonds (8 pi electrons) and a 2- negative charge (two lone pairs, 4 electrons) which participate in the pi system. Total pi electrons = 8+4=12. According to Huckel's rule, 4n+2=10 when n=2. Cyclooctatetraene dianion is aromatic. All carbon atoms are part of the conjugated system and each forms two sigma bonds and is part of the pi system. The two extra electrons are delocalized in the pi system. Each carbon atom is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
-
Cycloheptatrienyl cation (Tropylium cation): This is a cyclic compound with 7 carbon atoms and a positive charge. It has three double bonds, contributing 6 pi electrons. The carbon atom with the positive charge is bonded to two other carbon atoms and has an empty p orbital. The pi system consists of the 6 electrons from the double bonds. According to Huckel's rule, 4n+2=6 when n=1. Tropylium cation is aromatic. The carbon atoms in the double bonds are sp2 hybridized. The carbon atom with the positive charge forms three sigma bonds and has an empty p orbital. Thus, it is sp2 hybridized. So, this compound is aromatic and has only sp2 hybridized atoms.
All 10 given compounds are aromatic and have only sp2 hybridized atoms.
