Question
Question: Among the following, the molecule of highest dipole moment is: A. \(\text{CC}{{\text{l}}_{4}}\) ...
Among the following, the molecule of highest dipole moment is:
A. CCl4
B. NH3
C. H2O
D. CHCl3
E. BF3
Solution
Dipole moment is defined as the product of the charge present on the molecule and the distance between the two charges. More will the polarity of the molecule more will be its dipole moment.
Complete Answer:
- In the given question, we have to identify the molecule which has the highest value of dipole moment.
- As we know that dipole moment is the product of charge and distance between the two charges. And it is also considered as a vector unit because it depends on both magnitude and direction of the molecule.
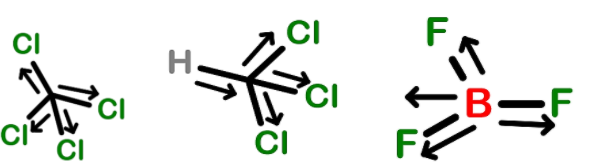
- So, among the given options the dipole moment of carbon tetrachloride, chloromethane and boron trifluoride has the zero-dipole moment.
- This is due to the symmetrical shape of the molecules due to which the dipole moment of one bond is cancelled by the dipole moment of the other bond due to the opposite charge and direction.
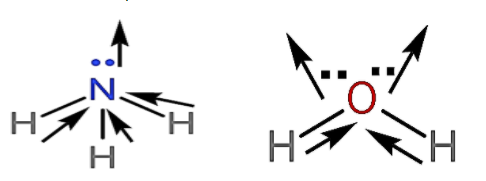
- Now, in the case of ammonia and water, water has the highest dipole moment.
- Because as we know that oxygen is more electronegative than nitrogen so it will have more attraction power than the nitrogen atom.
- Due to which the polarity or charge on the oxygen atom is more than the nitrogen atom. Hence, water has more dipole moment than ammonia.
Therefore, option C is the correct answer.
Note:
With help of the dipole moment we can also determine the stability of the molecule because higher dipole moment tells that the molecule is ionic and ionic molecules have more ability to react than the covalent character. So, lower will be the stability if the dipole moment of a molecule is high.
